Nobel Biocare N1™ concept in the posterior maxilla with sinus floor elevation
Dr. Oded Bahat, Dr. Ion Zabalegui, Dr. Eva Berroeta, Mr. Javier Pérez
Spain
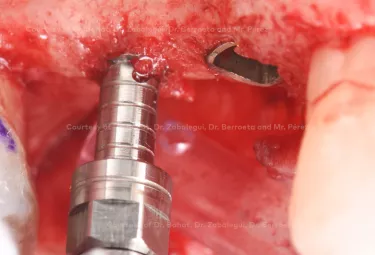
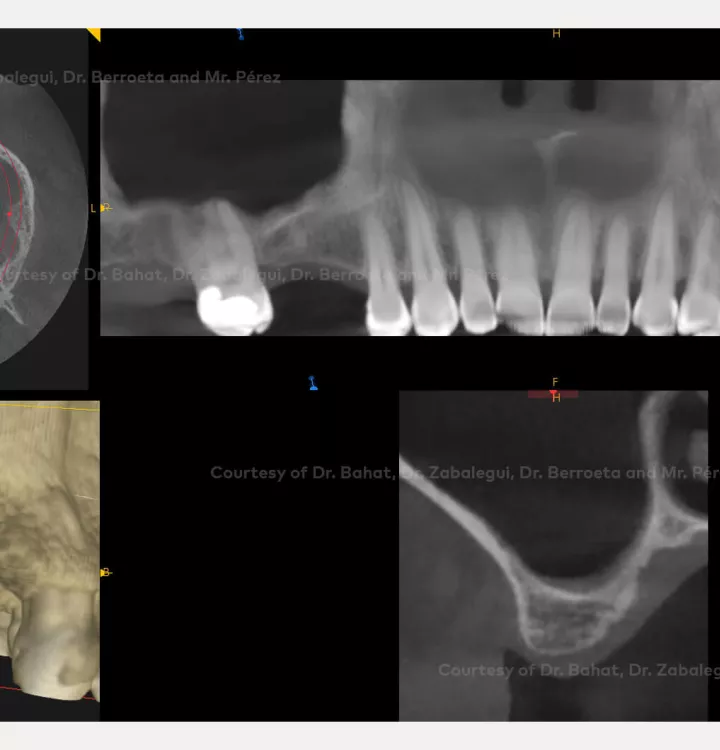
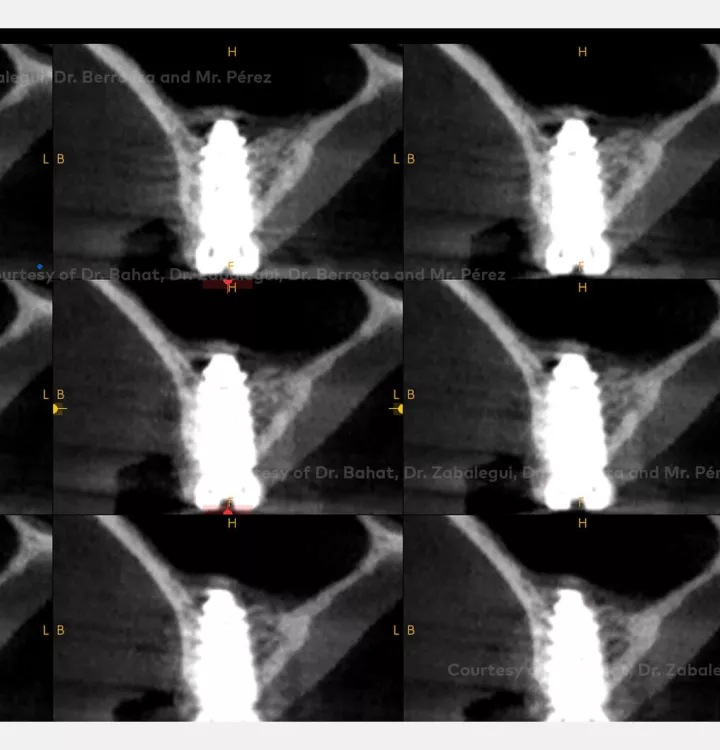
Implant placement associated with immediate sinus floor elevation at sites with inadequate vertical bone height.
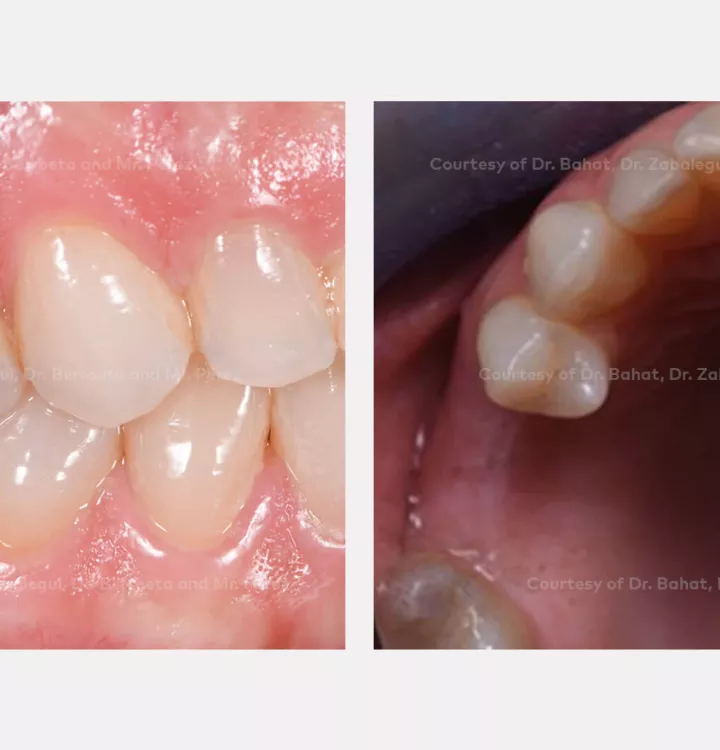
Patient: female, 47 years
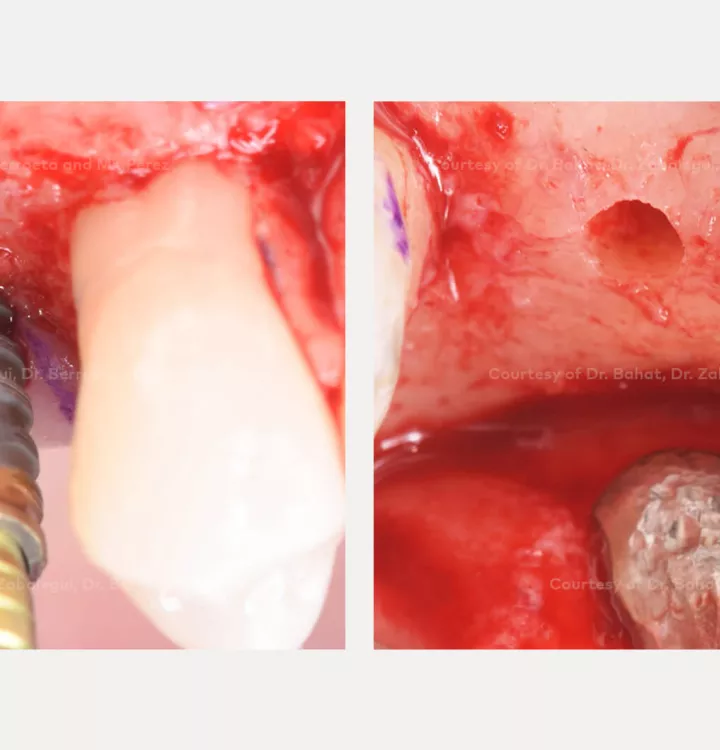
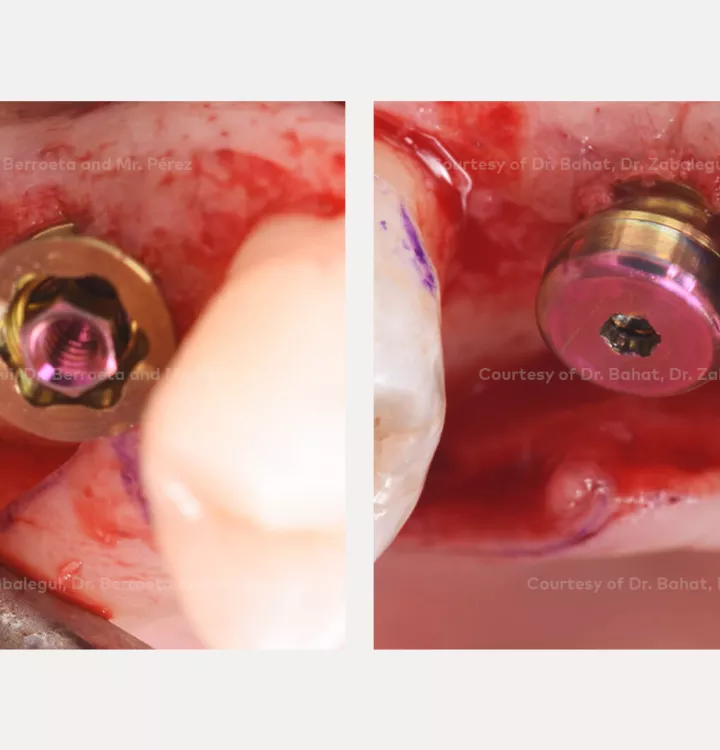
Clinical situation: healed site at tooth position 15, 16 (FDI) with bone resorption
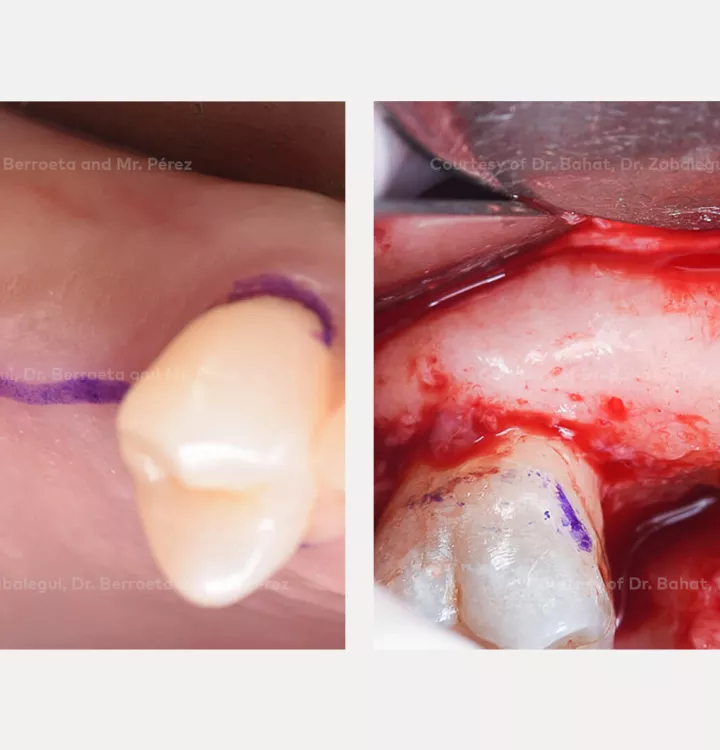
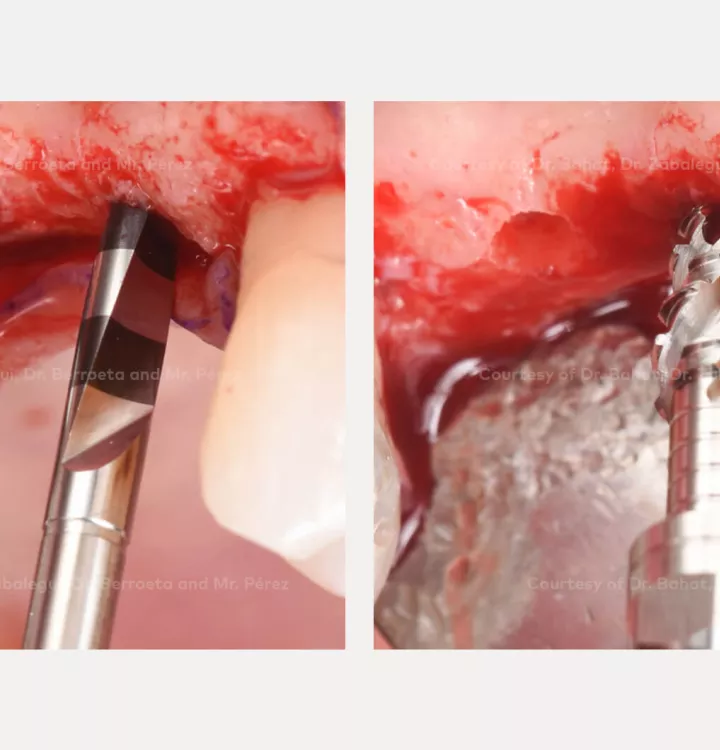
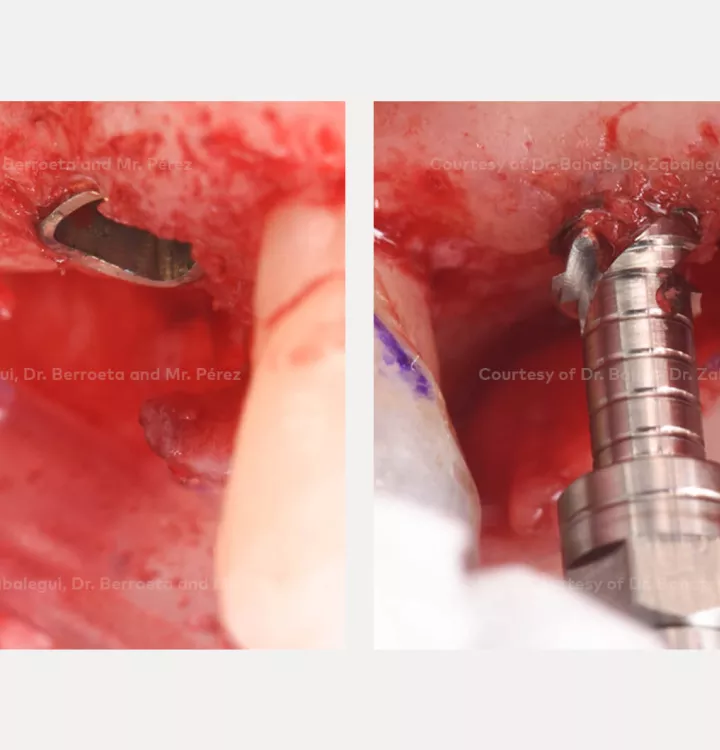
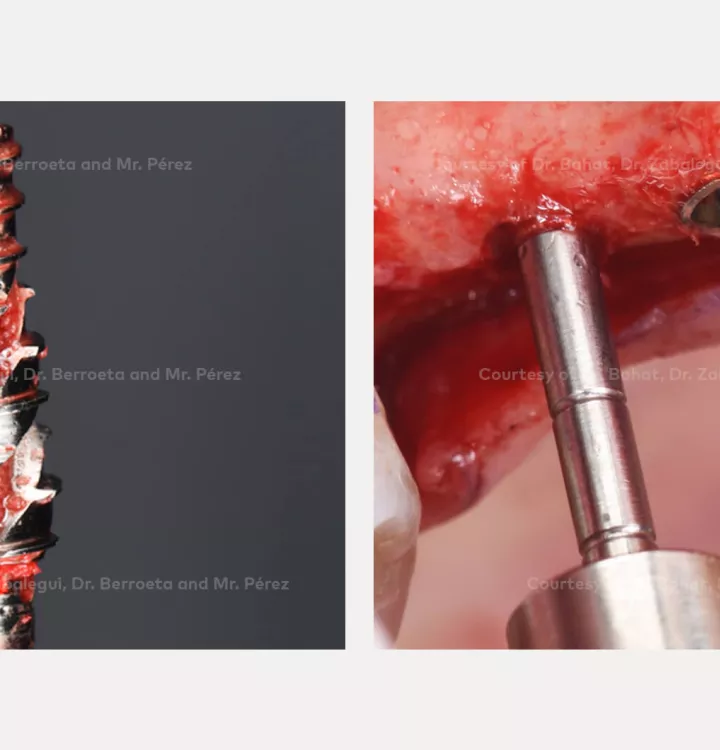
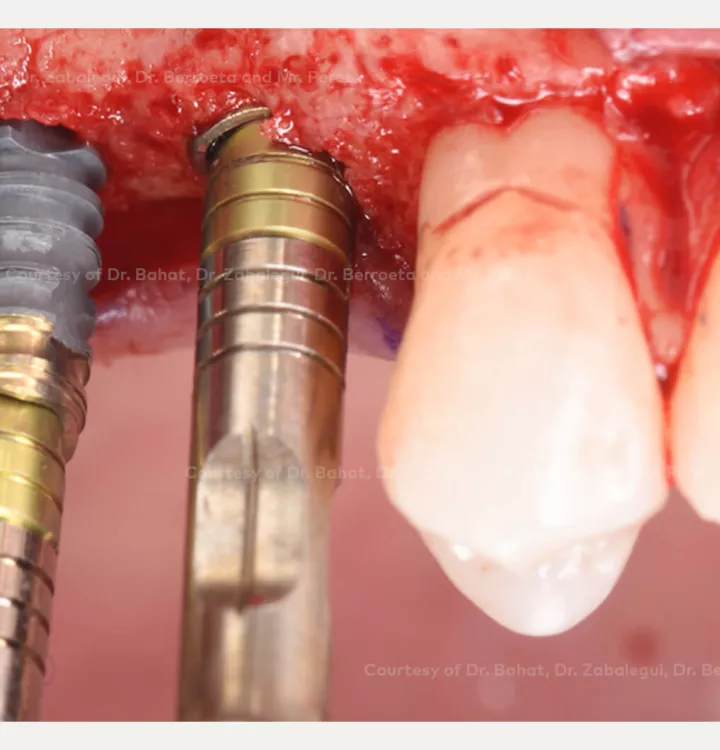
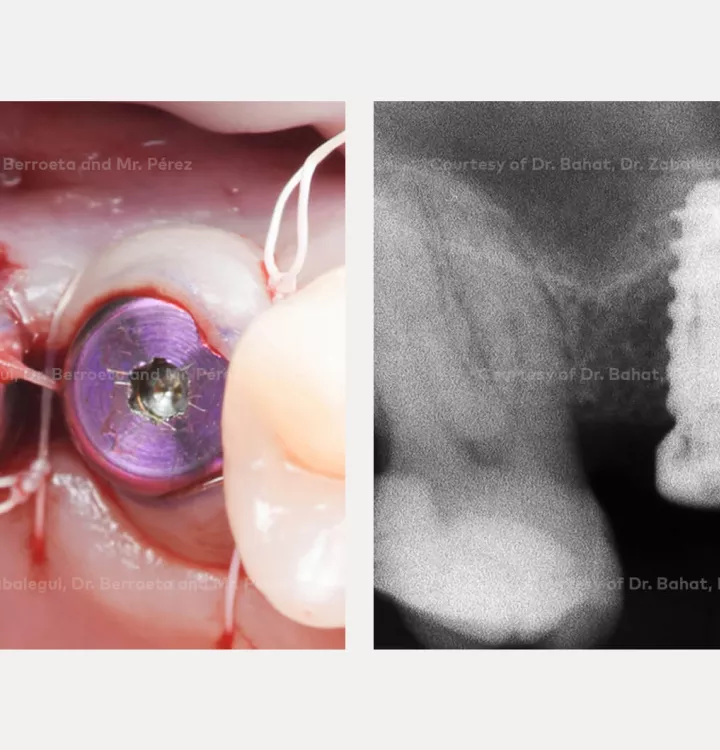
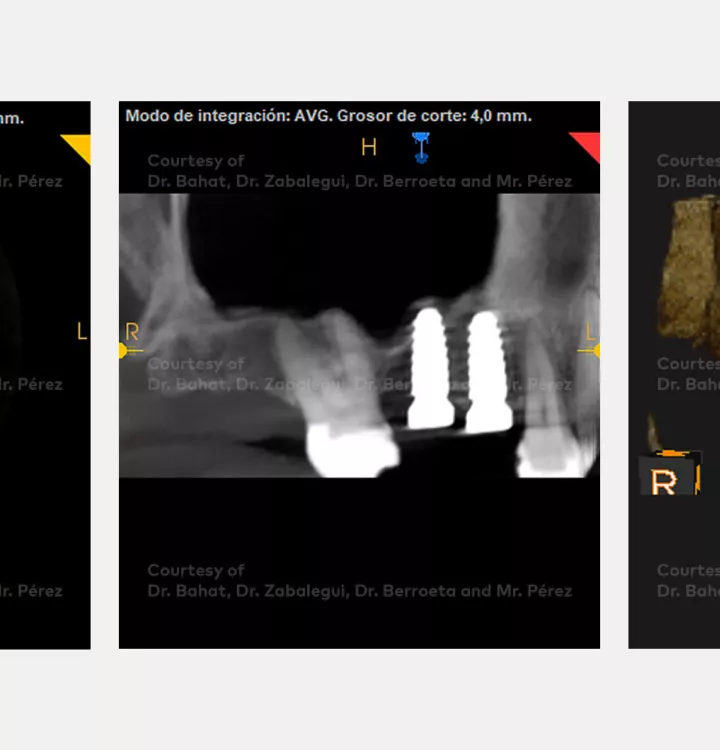
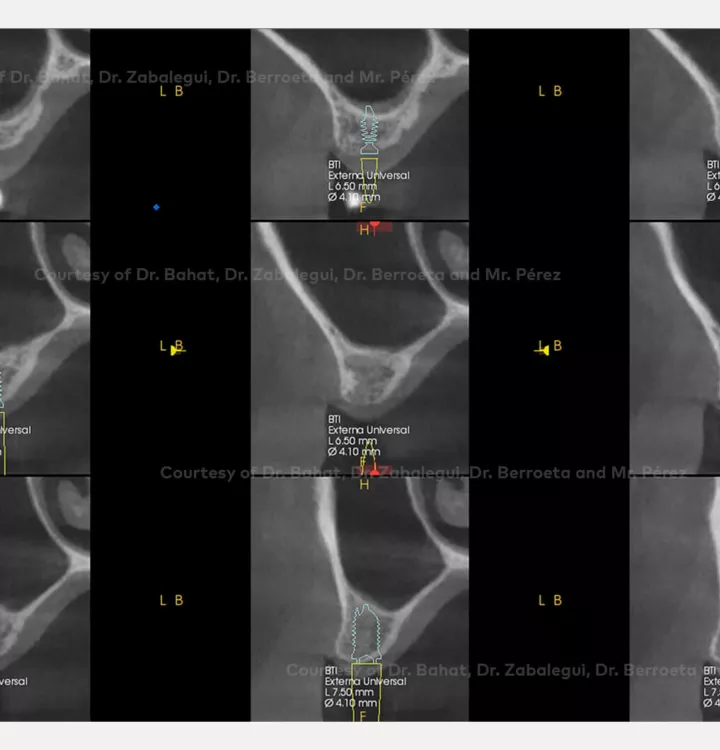
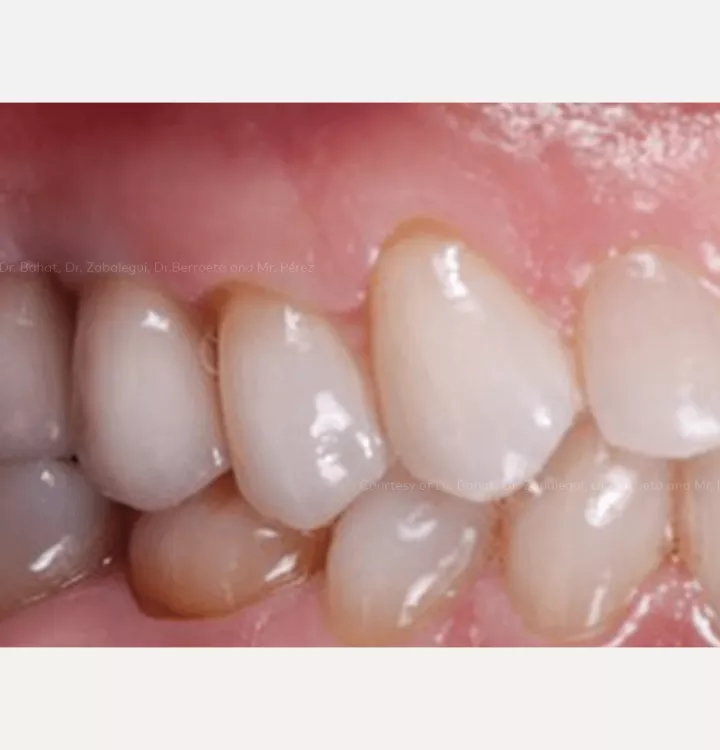
Surgical solution: placement of two implants with simultaneous sinus floor elevation
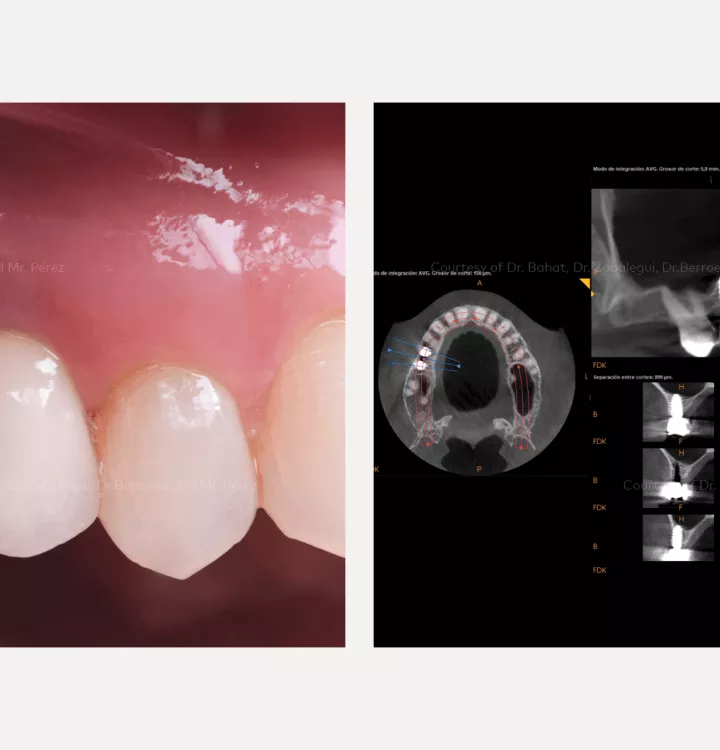
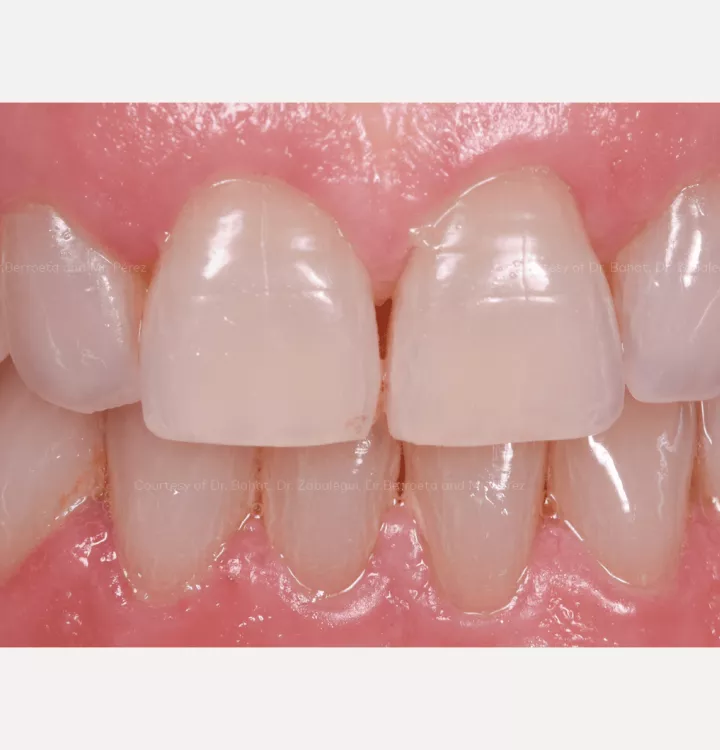
Restorative solution: 2 single crowns
Surgery date: Bilbao, April 25, 2019
Total treatment time: 12 months
Tooth position: 15, 16 (FDI) 3,4 USA
-
 Nobel Biocare N1™ system
Nobel Biocare N1™ systemChange the way you treat patients.
Sign up for our blog update
Get the latest clinical cases, industry news, product information and more.
© Nobel Biocare Services AG, 2019. All rights reserved. Nobel Biocare, the Nobel Biocare logotype and all other trademarks are, if nothing else is stated or is evident from the context in a certain case, trademarks of Nobel Biocare. Please refer to nobelbiocare.com/trademarks for more information. Product images are not necessarily to scale. Disclaimer: Some products may not be regulatory cleared/released for sale in all markets. Please contact the local Nobel Biocare sales office for current product assortment and availability. For prescription use only. Caution: Federal (United States) law restricts this device to sale by or on the order of a licensed clinician, medical professional or physician. See Instructions For Use for full prescribing information, including indications, contraindications, warnings and precautions. Nobel Biocare does not take any liability for any injury or damage to any person or property arising from the use of this clinical case. This clinical case is not intended to recommend any measures, techniques, procedures or products, or give advice, and is not a substitute for medical training or your own clinical judgement as a healthcare professional. Viewers should never disregard professional medical advice or delay seeking medical treatment because of something they have seen in this clinical case. Full procedure is not shown. Certain sequences have been cut.