You deserve
real science

We question the fairness of the study's comparisons and the validity of their marketing
Biased comparison
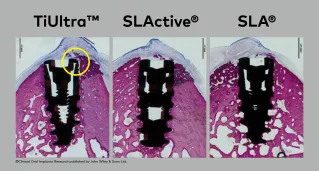
Do you know that in this study, the NobelActive TiUltra™ implants did not have original cover screws?
In the images, you can see the gap introduced by the use of a non-original cover screws with NobelActive® TiUltra™. The gap between the implant and the abutment/cover screw can lead to bacterial leakage, resulting in bone loss.3,4
Here's what happens when a non-original screw is used on an implant.
Read between the lines.
The measurements and marketing don't match.
Straumann's marketing claims that their graphics illustrate the measured bone growth (white lines). However, they do not correlate with the results that were actually published.
We’ve added the correct new bone height (NBH) results in red, revealing that the differences between SLActive® and TiUltra™ were exaggerated.
Underpowered study
Only one study, with no more than eight samples per group
As basic statistical principles indicate, the sample size is too small for a reliable comparison.6 Therefore, the study is underpowered. So, the study conclusion, “significantly superior levels of osseointegration (dBIC) and coronal bone apposition (fBIC) in the dehiscence defect” in SLA® and SLActive® groups cannot be substantiated.
Statistically non-significant endpoints
There was no statistical significance of multiple endpoints, including New Bone Height (NBH) and Bone Area to Total Area (BATA). These results are notably absent from Straumann’s marketing.
Incorrect statistical method
Even more concerning, the statistical methods used were not appropriate for several endpoints, and no correction was made for multiple testing across 10 interdependent measures—dramatically increasing the likelihood of false positives.
In a study where sample sizes are too small, non-original components are used, key endpoints are statistically non-significant, and incorrect statistical methods are used, do you have confidence in their conclusions?
Nobel Biocare believes you and your patient deserve the real science.
-
 Clinical outcomes with TiUltra™ surfaces
Clinical outcomes with TiUltra™ surfacesRead the latest data on TiUltra™ surfaces, featuring more than 1,270 patients, 2,000 implants, and 12 clinical studies.
-
 Sciconnect
SciconnectGet the latest updates on implant dentistry science, our solutions, innovations and educational events. SciConnect is where patient-centric innovations and clinical science meet.
-
 Our Story
Our StoryNobel Biocare is about you, for your patient. That’s a promise based on over 65 years’ R&D in implantology
References
1.Shahdad S, Bosshardt D, Patel M, et al. Benchmark performance of anodized vs. sandblasted implant surfaces in an acute dehiscence type defect animal model. Clin Oral Implants Res. 2022 Nov;33(11):1135-1146.
2. Shahdad S, Rawlinson S, et al. Osseointegration of Anodized vs. Sandblasted Implant Surfaces in a Guided Bone Regeneration Acute Dehiscence-Type Defect: An In Vivo Experimental Mandibular Minipig Model. Clin Oral Implants Res. 2024 Oct 10. doi: 10.1111/clr.14369. Epub ahead of print. PMID: 39387129.
3. Zipprich H, Miotke 5, Hmaidouch R, Lauer HC. A new experimental design for bacterial microleakage investigation at the implantabutment interface: an in vitro study. Int J Oral Maxillofac Implants 2016;31(1):37-44.
4. Karl M, Taylor TD. Effect of cyclic loading on micromotion at the implant-abutment interface. Int J Oral Maxillofac Implants, 2016;31(6):1247-1263.
5. Sasada Y, Cochran DL. Implant-Abutment Connections: A Review of Biologic Consequences and Peri-implantitis Implications. Int J Oral Maxillofac Implants. 2017 Nov/Dec; 32(6):1296-1307.
6. Lau F, Kuziemsky C, editors. Handbook of eHealth Evaluation: An Evidence-based Approach [Internet]. Victoria (BC): University of Victoria; 2017 Feb 27. PMID: 29431951.