Why you should not reuse a healing abutment
Healing of the soft tissue in the oral cavity has been under thoughtful study recently. In this article, the authors explain the significant influence the healing abutment has on that process.
The healing cap protects the internal aspects of the implant from debris accumulations and serves as the initial transmucosal connection between the external environment and the inner parts of the human body. As a bacteriological barrier with a tight connection between the epithelium and implant component, it helps to prevent infection, crestal bone loss and soft tissue recession, all of which are crucial for long term success.1
Healing abutment and the soft tissue barrier
The soft tissue barrier that contacts the standard titanium healing abutment consists of two zones:
1. A marginal zone consisting of junctional epithelium.
2. A deeper apical zone comprised of a fiber-rich connective tissue.
It has been shown that the properties of the material placed in contact with the soft tissues have a decisive influence on the quality of the mucosal attachment. Chemical composition and surface topography of the abutment material play a role in tissue recession and prevention of crestal bone loss. The ability of the cells to attach and spread is dependent upon surface hydrophilicity (wettability) and “lack” of surface contamination.
Although designed and labeled for single use, some clinicians advocate re-using—or “recycling”—healing abutments from one patient to the next for purely economic reasons. A breach of manufacturer guidelines, this is not a wise choice.
Five reasons why healing abutments are for single use only

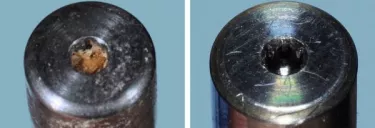
Figure 1. Healing abutment (left) was placed in an Ultrasonic bath for ten minutes, then autoclaved. However, since proper cleaning was not achieved, sterilization was not possible. Compare this to a new healing abutment (right) and the difference is obvious!
1. According to Nobel Biocare guidelines, the company’s healing abutments should each only be used once.
The argument against re-using a healing abutment is evident in the images above (Fig 1). No matter the method used—steam or chemical autoclave, ultra-violet light, or ethylene oxide—sterilization can never completely recreate the pristine surface of the original abutment.
2. Re-using a healing abutment labeled for single use may seriously degrade the performance of the product.
Sterilization with a steam autoclave, chemical autoclave, lasers or ethylene oxide may alter the composition of the titanium surface, negatively affecting cells.
Physical surface topography changes the titanium wettability, which interferes with the epithelium and fibroblast cells’ ability to attach and spread. This effect is quite different from that of a new (i.e. not previously used) healing abutment.
3. New screw threads are vital for consistently favorable results.
The screw thread component of the healing abutment may also contain bio-burden after it has been removed, and a screw thread is by far the most difficult part of the healing abutment to clean (Fig. 2).
Although not in direct contact with healing tissue, studies have confirmed contamination and wear affect the screw thread and may result in damage within the implant.
Contamination can also lead to healing abutments “locking” onto the implant. This is an extreme issue and has been known to result in the implant being reverse-torqued out of the bone in attempts to unscrew the abutment.
4. The screwdriver may not properly engage a re-used healing abutment.
Very difficult-to-remove debris (Fig 3) may clog the screw driver insertion site. It should be noted that ultrasonic baths are not likely to get rid of this tightly packed material. Also, with repeated use, the screw head itself can become mechanically damaged.
Case reports in the literature report that such damage—unnecessary if you follow the manufacturer’s single-use guidelines—makes healing abutment retrieval problematic (Fig. 4).
5. Mechanical cleaning of a previously used abutment—especially via air particle abrasion—can damage the abutment/implant connection.
Thus reducing its sealing capacity and altering the component connection (Fig. 5 A & B). Studies have also reported abrasive impregnation into the softer titanium, resulting in metal contamination.

Figure 5. Scanning electron microscopy images of conical connection at 500x magnification (A – to the left). The effect of air particle abrasion cleaning on the fitting surface of an implant abutment is clearly evident (B – to the right). The implant-abutment interface with a new, clean abutment surface for comparison.
A potentially costly risk
Take these five points under careful consideration and you will find that the potential monetary savings of re-using a healing abutment a second time do not outweigh the known and potential health risks to the patient.
In short: in order to provide patients with the greatest chance of soft tissue attachment, minimized inflammation and the prevention of possible recession—and to give the bone a healthy start—always use a clean new healing abutment!
References
1. Altankov G, Grinnell F, Groth T. Studies on the biocompatibility of materials: fibroblast reorganization of substratum-bound fibronectin on surfaces varying in wettability. J Biomed Mater Res. 1996 Mar;30(3):385-91.
An N, Rausch-fan X, Wieland M, Matejka M, Andrukhov O, Schedle A. Initial attachment, subsequent cell proliferation/viability and gene expression of epithelial cells related to attachment and wound healing in response to different titanium surfaces. Dent Mater. 2012 Dec;28(12):1207-14.
Bousquet P, Bennasar IC, Tramini P, Jacquemot M, Cuisinier F. Tightening of healing abutments: influence of torque on bacterial proliferation risk, an in vitro investigation. Biomed Tech (Berl). 2014 Dec;59(6):495-500.
Canullo L, Penarrocha-Oltra D, Marchionni S, Bagán L, Peñarrocha-Diago MA, Micarelli C. Soft tissue cell adhesion to titanium abutments after different cleaning procedures: preliminary results of a randomized clinical trial. Med Oral Patol Oral Cir Bucal. 2014 Mar 1;19(2):e177-83
Darvell BW, Samman N, Luk WE, Clark RK, Tiderman H. Contamination of titanium castings by aluminium oxide blasting. J Dent. 1995;23(5): 319-3
Ntrouka V, Hoogenkamp M, Zaura E, van der Weijden F. The effect of chemotherapeutic agents on titanium-adherent biofilms. Clin Oral Implants Res. 2011 Nov;22(11):1227-34.
Ruardy TG, Schakenraad JM, van der Mei HC, Busscher HJ. Adhesion and spreading of human skin fibroblasts on physicochemically characterized gradient surfaces. J Biomed Mater Res. 1995 Nov;29(11):1415-23.
Vezeau PJ, Keller JC, Wightman JP. Reuse of healing abutments: an in vitro model of plasma cleaning and common sterilization techniques. Implant Dent. 2000;9(3):236-46
Vezeau PJ, Koorbusch GF, Draughn RA, Keller JC. Effects of multiple sterilization on surface characteristics and in vitro biologic responses to titanium. J Oral Maxillofac Surg. 1996 Jun;54(6):738-46
Welander M, Abrahamsson I, Berglundh T. The mucosal barrier at implant abutments of different materials. Clin Oral Implants Res. 2008 Jul;19(7):635-41