“These results are a very pleasant surprise”. New study findings of full-arch treatment with new surfaces
– 100% cumulative implant survival and prosthetic success
– Low marginal bone remodeling
– Stable and healthy soft tissue response
–No biological complications
As the MALO CLINIC’s Director of Research, Development and Education and Oral Hygiene, Dr. Miguel de Araújo Nobre has more than 20 years of experience as a clinical researcher under his belt. In this interview, he outlines the promising 1-year results of a prospective clinical study of Nobel Biocare’s Xeal abutment surface and TiUltra implant surface for All-on-4® treatment.1 The study is being conducted by Dr. Nobre and Dr. Ana Ferro, the Head of the MALO CLINIC’s Oral Surgery Department, who presented the results at the European Association for Osseointegration’s Digital Days.
Dr. Nobre, please tell us a bit about yourself and your professional background
I graduated as a dental hygienist in 1998 and started working at the MALO CLINIC in 1999 first as a dental hygienist, and then in research, development and education. My first published study was in 2003 on the All-on-4® treatment concept and, since then, I have co-authored over 100 scientific publications in international peer-reviewed journals alongside 6 book chapters. Along the way, I completed an MSc and a PhD at the University of Lisbon’s Faculty of Medicine, where I am now a visiting professor in oral pathology and oral and maxillofacial surgery. Working at the MALO CLINIC is a lot of fun – I have the opportunity to work both on the scientific research element of my career but also in the field treating patients with implant-supported rehabilitations.
What is the aim of this Xeal and TiUltra surface study?
The aim of the study is to evaluate the clinical outcomes, at 3 years, of full-arch rehabilitations conducted with the All-on-4® treatment concept, using NobelParallel CC implants with the TiUltra surface and Multi-unit Abutments (MUAs) with the Xeal surface. It’s an ongoing study and there were some difficulties setting it up due to the pandemic, but we’ve now published the pilot study results at the 1-year follow-up.
Can you briefly explain the materials and the methods used?
It’s a prospective single-cohort pilot study with 16 patients in full-arch rehabilitation, and in total, 64 implants were placed, all of which were NobelParallel CC TiUltra and 64 MUAs with Xeal. These were all Immediate Function implants supporting 16 full-arch prostheses — 11 in the maxilla and 5 in the mandible. These patients were treated following our maintenance protocol, meaning clinical appointments during the functional osseointegration period and after 10 days, 2 months, 4 months, 6 months, and 1 year. We then analyzed prosthetic success rates and implant survival as primary outcome measures while also examining marginal bone loss, biological and mechanical complications, the plaque index and the bleeding index as secondary outcome measures.
And what were the key results?
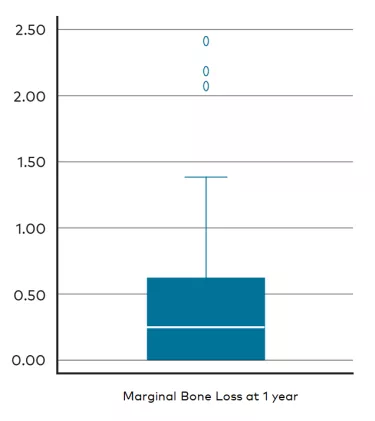
We achieved a 100% survival rate for both implant and abutment with a mean marginal bone loss of just 0.46 mm after 1 year. Though some mechanical complications occurred with provisional prosthesis screw fractures, there was an absence of biological complications and a good and stable soft tissue response. In a nutshell, these are good results.
Though it’s a pilot study with one-year follow-up, is this 100% survival rate a very promising figure?
Through our experiences with All-on-4® Immediate Function, we have learned over the years that if a stable maintenance protocol is kept up, the majority of implant failures normally occur in the first year or two of follow-up. We also know that the osseointegration period is an important milestone for immediate function implants and, once it is passed, the probability of implant failure is low. In my opinion, to register a 100% survival during the first year of follow-up is very promising. From a researcher’s point of view, the one-year survival rate is an important indicator of potential long-term success. Yes, it’s still a pilot study with 16 patients and it’s still quite early, but as we’ve discovered with All-on-4®, a one-year outcome like this gives you a positive start.
Regarding the marginal bone loss results: What can a clinician learn from this? Is there any indication for potential long-term outcomes?
Often the amount of marginal bone loss is a predictor of long-term outcome success. When you place an implant, the implant failure likelihood tends to decrease over time but at the same time, the probability for pockets of marginal bone loss increase. This is the difference between early and late implant failure. For this study, we recorded an average of 0.46 mm of marginal bone loss after 1 year—0.57 mm in the maxilla, and 0.19 mm in the mandible. From our perspective, these results are a very pleasant surprise. It was less than we are used to observing from the All-on-4® concept and though we can’t directly compare this with data from older studies, in this case we are looking in-house and so it’s a very promising result.
What made you first adopt the Xeal and TiUltra surfaces?
First of all, we have previous experience using anodized surfaces — we started using Nobel Biocare’s TiUnite implant surface a long time ago, and have up to 18 years’ follow-up data that prove they are a reliable long-term option.
We are always trying to improve the treatment quality for our patients, who constantly pose rehabilitation challenges. These improvements can usually either be achieved through updated protocols or the use of new innovations. For these new Xeal and TiUltra surfaces, we scrutinized the research beforehand. We were particularly interested in the Mucointegration™ concept; from our view, it fills a gap in implant dentistry in relation to the peri-implant complex.
Example clinical case from the study: A 53-year-old female with failing dentition and severe bone atrophy in the maxilla was treated according to the All-on-4® treatment concept with four NobelParallel CC TiUltra implants and Multi-unit Abutments Xeal. A temporary prosthesis, Malo Clinic all-acrylic bridge, was placed on the day of surgery while the final prosthesis, Malo Clinic acrylic bridge on NobelProcera implant bar, was placed 3 months later. At 1 year, the patient shows excellent soft tissue health around the final restoration.
References
1. Ferro A, De Araújo Nobre M. All-on-4 concept using TiUltra surface implants and Multi-unit Xeal abutments: Pilot study report. Clin Oral Implant Research 2021; 32(S22):59.