
A metal-free solution for restoring natural esthetics
Dr Jens Tartsch, Switzerland.
This article is taken from the third-party journal, Ceramic Implants 02/2019.
Ceramic dental implants have established a strong presence in general implant dentistry. Patient demand for metal-free solutions is increasing, and the development of new materials, micro-rough surfaces and improved treatment protocols has enabled clinicians to use ceramic dental implants as a reliable treatment alternative to titanium implants. The patient should be informed about the advantages and disadvantages of both material options and involved in decision-making if a ceramic implant is presented as a treatment option. This procedure is standard in our dental office, shown by the following case report.
Clinical situation and treatment planning
A 57-year-old female patient presented at our clinic in May 2018. She asked for restoration of her premolar and molar sites after extraction. The teeth had been extracted elsewhere three months (tooth #45) and one year (tooth #46) before her visit (Figs. 1a & b). The radiographic examination showed a single lateral dehiscence defect at site #45 and a fully healed site #46 (Fig. 2). The patient was informed about ceramic implants as an alternative to titanium implants and the NobelPearl™ tapered dental implant system (Nobel Biocare) as a metal-free solution. After a detailed explanation and discussion, the patient decided on this treatment option. The main reason for her decision was the prognosis of less inflammation of the peri-implant tissue with ceramic implants. The disadvantage of less long-term evidence compared with titanium implants was taken into account. After an initial hygiene phase and the periodontal treatment of tooth #47 by root planing, the implant surgery was performed in July 2018.
-
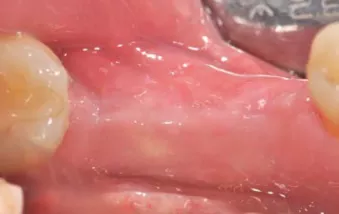
Figs. 1a: Healed sites after extraction of teeth #45 and 46, three months before surgery.
-
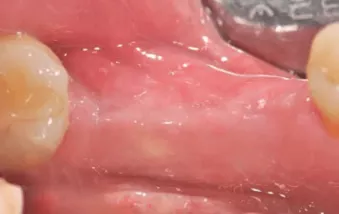
Figs. 1b: Healed sites after extraction of teeth #45 and 46, one year before surgery.
-
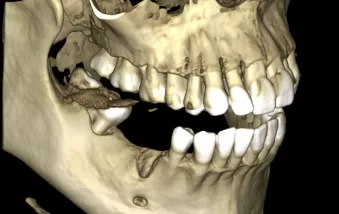
Fig. 2
Surgical and restorative protocols
After cleaning of the alveolar bone, a suitable bone level for implant insertion in site #46 was established and a lateral defect at site #45 was observed (Fig. 3). NobelPearl tapered dental implants of 4.2 mm in diameter and 10.0 mm in length with NobelPearl Inter-X straight abutments were used in the clinical procedure (Fig. 4). Both implants were inserted 0.6 mm supra-crestally at a torque of 30 Ncm. Primary stability was good. The implants were covered with Inter-X cover screws in situ (Fig. 5).
-
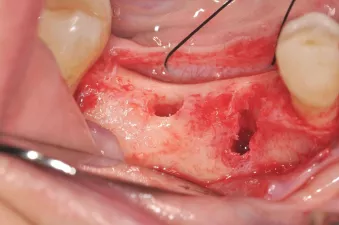
Fig. 3: Suitable bone for implant insertion in site #46 and a lateral defect at site #45
-
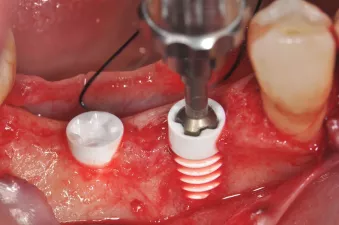
Fig. 4: Manual insertion of a NobelPearl implant using the NobelPearl Inter-X implant driver after preparation of sites.
-
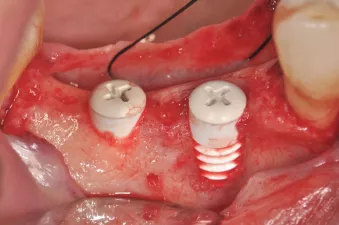
Fig. 5: NobelPearl Inter-X cover screw in situ.
The buccal surface of implant #45 remained within the bone contour (three-wall defect), thus the lateral defect could be easily augmented with deproteinized bovine bone (Bio-Oss®, Geistlich) and a membrane (Bio-Guide®, Geistlich) following the standard clinical procedure (Figs. 6 & 7). After smoothening a periosteal incision, primary wound closure could be achieved. Three months after surgery, the restorative process began (Figs. 8–10) with re-entry by small single roll flaps at each implant site and placement of 3 mm NobelPearl healing abutments. The soft tissue was healthy and keratinized around the healing abutments when open-tray impression taking was performed after two weeks. A monolithic zirconia crown was selected as the prosthetic solution. For stability, and because of the augmentation at site #45, the crowns were splinted.
-
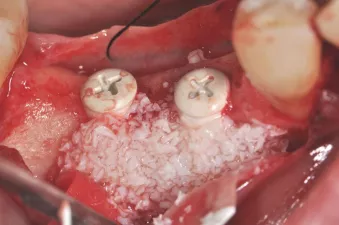
Fig. 6: Horizontal augmentation using deproteinized bovine bone.
-
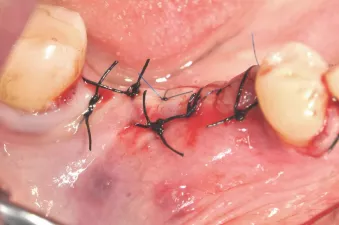
Fig. 7: With a two-piece implant system, primary wound closure is possible.
-
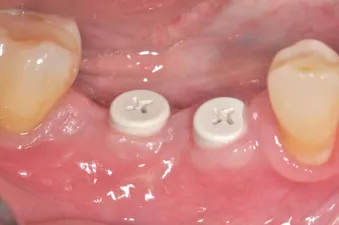
Fig. 8: Healthy soft tissue two weeks after re-entry.
-
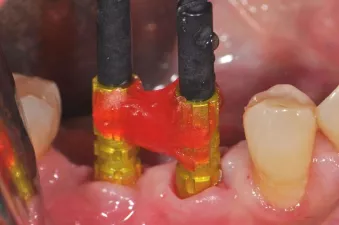
Fig. 9: NobelPearl Inter-X open-tray impression coping placed on implants and fixed for precision.
-
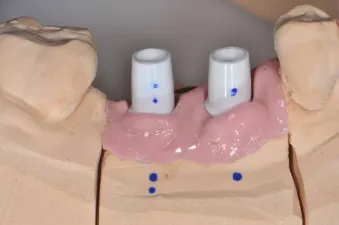
Fig. 10: Abutments placed on the master cast with a gingiva mask.
After removal of the healing abutment (Fig. 11) and try-in of the NobelPearl abutments, the screw channels were sealed with Teflon and the abutments were prepared for cementation (Figs. 12–14). To achieve a tension and bending-free connection between the restorations and the implants, the zirconia restorations were cemented intra-orally to the abutments according to the standard procedure using RelyX Unicem (3M ESPE). Teflon was removed through the screw channel and the entire restoration was removed again as a whole piece. The cement could then be easily removed and the final restorations polished. After this cleaning, the restorations were reinserted and the VICARBO screws were torqued to 25 Ncm according to the guidelines. The screw channels were closed again using Teflon tape (Fig. 15) and finally covered with composite (Figs. 16 & 17). The total treatment time was four months.
-
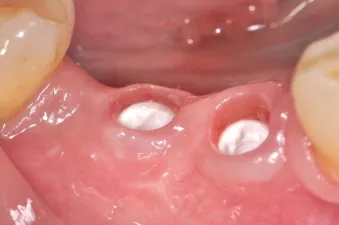
Fig. 11: Healthy and thick keratinized peri-implant mucosa seen after removal of the NobelPearl Inter-X healing abutment.
-
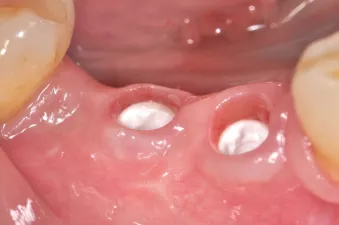
Fig. 12: The screw channels were sealed with Teflon and the NobelPearl abutments prepared for cementation.
-
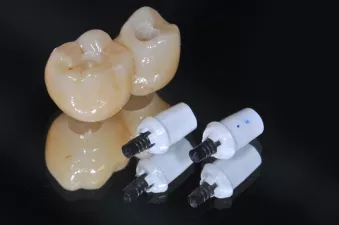
Figs. 13: Final restorations and abutments before insertion.
-
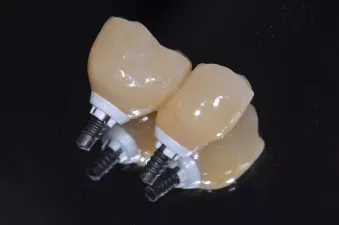
Fig. 14: Final restorations and abutments before insertion.
-
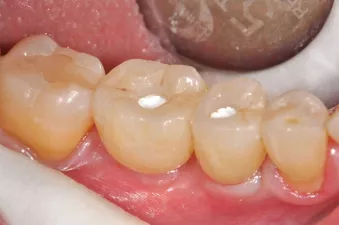
Fig. 15: For the final installation of the restorations, the screws were torqued to 25 Ncm and the screw channels were closed again with Teflon tape.
-
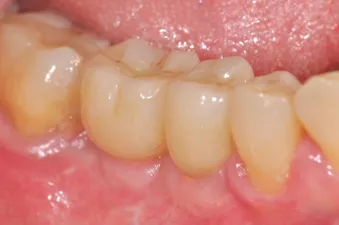
Fig. 16: Final restorations in situ after screw channel closure and sealing with composite.
-
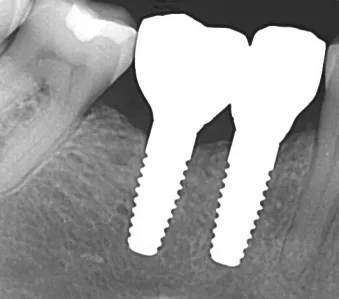
Fig. 17: Control radiograph after delivery of the restorations.
Clinical outcomes
The result was beautiful, and the patient was highly satisfied at the one-year follow-up (Figs. 18 & 19). No inflammation or prosthetic problems occurred during the follow-up period. The result in this case was a metal and cement-free, screw-retained and reversible restoration. If problems such as chipping or subsequent colour adjustments were to become obvious, these could be easily corrected and revised—like with titanium implants.
-
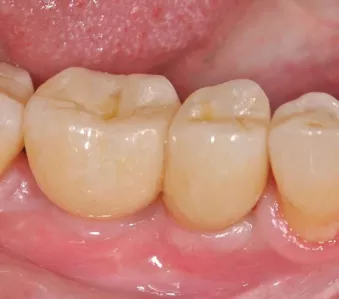
Fig. 18: Excellent aesthetics at the one-year follow-up. Note the healthy soft tissue and natural-looking restorations.
-
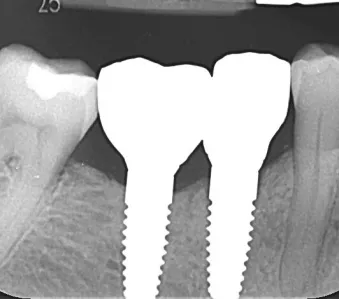
Fig. 19: The one-year follow-up radiograph showed a stable bone level.
Conclusion
The NobelPearl tapered dental implant system is designed for a broad range of indications, from single units to multiple units. It performed extremely well in the case presented, carrying splinted crowns after bone augmentation of defective bone. The surgical and prosthetic protocols are comparable to those of titanium implants. These are important factors for the successful integration of a new dental implant system in the daily dental practice. Our main reasons for using the NobelPearl tapered dental implant system in the case presented were as follows:
– NobelPearl is designed to support a natural soft-tissue appearance, especially for patients with a thin mucosal biotype.1 Zirconia generally shows lower plaque accumulation2 and bacterial adhesion3,4 than does titanium.
– The surface of NobelPearl is micro-rough and hydrophilic for successful osseointegration, while the implant collar is partially machined, designed for excellent soft-tissue attachment and a low inflammatory response.5
– NobelPearl provides a mechanical strength It is made of alumina-toughened zirconia, which yields improved hardness, bending strength and toughness compared with tetragonal zirconia polycrystals.6
– NobelPearl offers great restorative flexibility owing to its two-piece reversible, cement-free internal connection
– The primary stability of the implant is good, as a result of having a non-self-cutting tapered body, and the clinical protocol is comparable to that of titanium implants.
– It is a metal-free solution, as even the screw is made of a carbon fibre-reinforced polymer designed for a strong ceramic-to-ceramic connection, which is highly biocompatible.
About the author

Dr Jens Tartsch received his degree in dentistry from Freie Universität Berlin in 1992. He practices at his own private dental clinic in Kilchberg in Switzerland. He is President of the European Society for Ceramic Implantology and a member of the board of the Swiss Society for Anti-Aging Medicine and Prevention. He is an international educator and published author on the topics of ceramic implantology and immunology in dentistry, as well as oral and maxillofacial surgery. His research and clinical interests are immunology in dentistry, ceramic implant dentistry and metal-free restorations, as well as aesthetic dentistry. He also lectures on national and international level.
References
GMT 64637 © Nobel Biocare Services AG, 2019. All rights reserved. Distributed by: Nobel Biocare. Nobel Biocare, the Nobel Biocare logotype and all other trademarks are, if nothing else is stated or is evident from the context in a certain case, trademarks of Nobel Biocare. Please refer to nobelbiocare.com/trademarks for more information. Product images are not necessarily to scale. Disclaimer: Some products may not be regulatory cleared/released for sale in all markets. Please contact the local Nobel Biocare sales office for current product assortment and availability. For prescription use only. Caution: Federal (United States) law restricts this device to sale by or on the order of a licensed dentist. See Instructions For Use for full prescribing information, including indications, contraindications, warnings and precautions.
1. Cosgarea R, Gasparik C, Dudea D, et al. Peri-implant soft tissue colour around titanium and zirconia abutments: a prospective randomized controlled clinical study. Clin Oral Implants Res 2015; 26(5):537–544.
2. Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol 2000 2017;73(1):241-258.
3. Scarano A, Piattelli M, Caputi S, et al. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol 2004;75(2):292-296.
4. Rimondini L, Cerroni L, Carrassi A, et al. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants 2002;17(6):793-798.
5. Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol 2000 2017;73(1):241-258.
6. Spies BC, Sauter C, Wolkewitz M, Kohal RJ. Alumina reinforced zirconia implants: effects of cyclic loading and abutment modification on fracture resistance. Dent Mater 2015;31(3):262-272