Overcoming obstacles
Dr. Helms discusses the molecular mechanisms of bone formation, implications for immediate implants and interesting directions for further long-term research.
Working with researchers Xing Yin, Jingtao Li, and Xibo Pei at the Stanford University Department of Surgery, Professor Jill A. Helms has been studying the biology and mechanics of immediately loaded implants. In this, the third of a three-part series on the topic, Dr. Helms discusses the molecular mechanisms of bone formation, implications for immediate implants and interesting directions for further long-term research.
In the previous two articles (see the “more to explore” section below) we discussed the extraction socket and what happens—from both biological and biomechanical perspectives—around implants that are placed in extraction sockets. In this third and final article, we focus on overcoming obstacles to accelerate implant osseointegration at extraction sites.
Of all the dental specialties, the field of implantology stands apart as a leader in design innovation. New materials, modifications in surface textures and implant shapes—as well as advances in osteotomy site preparation—have all contributed significantly to the success of dental implants. And yet there remains still more design space for improvements. In my laboratory at Stanford, our goal is to tweak the body’s natural bone-making capacity to accelerate bone formation around implants.
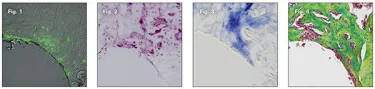
Some people wonder if new bone is actually necessary. After all, when you place an implant, and it’s stable, it’s reasonable to wonder why you might need new bone. The answer has to do with the fact that even the most careful implant site preparation still damages bone, and this damage can be exacerbated if an implant is placed with excessively high insertion torque. These types of damage are essentially “erased” from the bone because this tissue is in a constant state of turnover: dead osteocytes (Fig. 1) trigger bone resorption by osteoclasts (Fig. 2) and the void left behind needs to be filled with new bone (Fig. 3). It is this new bone that is ultimately responsible for the long-term osseointegration of an implant (Fig 4).
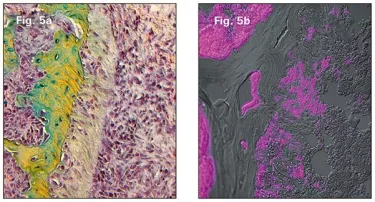
My colleagues and I started with a simple enough question: Where does this new osseointegrating bone come from? The answer is that the new bone arises from a population of bone-forming progenitor cells lining the socket, and are especially concentrated in the remnants of the periodontal ligament left behind after tooth removal (Fig. 5a). These osteo-progenitors are Wnt responsive (Fig. 5b).
Potent proteins
Some people may have heard of Wnt proteins acting as intercellular signals in other contexts, but for many readers this may be an unfamiliar word in the context of dentistry. Here’s the reason they are so interesting: Wnts are potent bone-inducing proteins. Patients with high bone mass diseases have too much Wnt signaling, while patients with osteoporosis have too little. In my lab, we see Wnts as a potential therapeutic agent to accelerate implant osseointegration.
Serious team science
The cells responsible for osseointegration are Wnt-responding cells, that is, they respond to the body’s own Wnt signal. We wanted to test whether or not delivering Wnt protein itself to the tissues around an implant could accelerate the formation of new bone. Proving this was a challenge. Even in rodent models, miniaturized dental implants routinely osseointegrate! Therefore, we had to recreate a condition where implants would reliably fail, and then potentially rescue that failure with the Wnt therapeutic. We created this scenario in an animal model by intentionally placing implants into oversized osteotomies.
What came next required some serious team science: biochemists had to purify the Wnt therapeutic and make it ready for in vivo use, bio-engineers had to characterize the mechanical environment, and an international group of talented surgeons had to devise methods to place the implants in sleeping mice.
As anticipated from human data, the loose-fitting implants in the rodents were surrounded by a persistent fibrous envelope. Using these unstable implant cases, one-half were treated with the Wnt therapeutic (delivered by injection into the fibrous tissue capsule) and the other half received an injection of saline. Then we waited.
The first sign we looked for was evidence that the Wnt therapeutic “activated” cells in the fibrous tissue envelope. Within 24 hours of injection, we saw the first signs that fibroblasts responded to the Wnt stimulus and began to multiply (Fig. 6a). Fibroblasts around the saline-treated implants showed no such response.
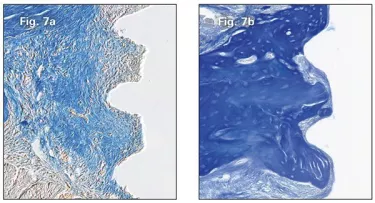
A few more days passed and the Wnt responsive osteoprogenitors continued to multiply (Fig. 6b and 6c). A few more days and the once-loose implants that had been treated with the Wnt therapeutic were encased in bone, whereas those treated with saline remained in a fibrous tissue envelope (Fig. 7b and 7a respectively). We succeeded in rescuing the failing implants!
We still have challenges ahead before a Wnt therapeutic is available for implant specialists. A bone anabolic agent must be safe after all. Some might remember issues surrounding use of Bone Morphogenetic Protein 2 (BMP2) in dental and orthopedic patients; these reported complications are unacceptable for non-life-threatening indications such as dental implants. The protein also must be manufactured to meet regulatory specifications. Finally, any new biologic must fill an important gap in clinical care.
Our research, and the biologics that are the fruit of that research, specifically address challenges to the osseointegration of immediate implants in routine clinical practice. The work continues.