Dental Implant Surface Treatments: What You Need to Know
When considering the factors that can affect dental implant success both long and short term, one of the most crucial is undoubtedly the implant’s surface.
Titanium has been long established as the standard bearer for dental implant material. However, when considering the factors that can affect dental implant success both long and short term, one of the most crucial is undoubtedly the implant’s surface.
In this article, we explain the importance of surfaces, compare different types of dental implant surfaces, and explore recent developments in anodized surfaces – for implants and abutments – that have been introduced with the Xeal™ and TiUltra™ surfaces.
- 1. Why dental implant surface matters
- 2. Developments in implant surface modification
- 3. Study comparing types of dental implant surfaces
- 4. Largest ever meta-analysis of a single implant brand
- 5. Entering the Mucointegration™ era: Developments in anodized surfaces, with X…
- 6. Smooth abutment surface for the Mucointegration™ process
- 7. More than roughness – The multi-zone TiUltra™ implant surface
Why dental implant surface matters
At first glance, titanium might seem a strange choice for dental implant material. Though it has high strength, great biocompatibility, low level of toxicity potential and high resistance to corrosion, titanium in its pure state is highly reactive.1 When it interacts with oxygen, however, a surface layer of titanium oxide (TiO2) is formed, stabilizing the surface and allowing osseointegration to occur.2
Surface has an important role in healing time for osseointegration and, ultimately, the success of implant treatment.3 It is the only part of the implant exposed to the surrounding oral environs, and its chemical, physical, mechanical, and topographic surface characteristics are all crucial to maximizing the likelihood of successful osseointegration.3
Developments in implant surface modification
Today, there is a wide range of titanium dental implant surfaces. P-I Brånemark’s initial implants over 50 years ago had a smooth, turned surface, and as he described, they require three to six months of healing before successful implant loading can take place.4
Since then, the design of dental implants and their surfaces have frequently changed and evolved over time to allow for improved osseointegration and better long-term implant survival rates. There are three distinct groups of methods through which implant surfaces can be modified at manufacture.
– Mechanical treatments: These include grinding, blasting and machining to create rougher or smoother surfaces.
– Chemical treatments: Conducted with acids, alkali, sol gel or through anodization, among other methods, chemical treatments alter the implant surface’s roughness and composition and enhance surface energy.5
– Physical treatments: These treatments include plasma spraying and ion deposition.
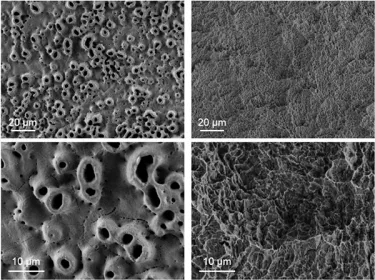
Some of the more common titanium implant surface treatments used in recent years include anodization, sand blasting and acid etching. Anodization, which works by increasing the thickness of the implant’s TiO2 layer, moderately roughening it, and improving osteoconductivity, has been shown to enhance osseointegration.6,7,8 Sandblasting and acid etching, on the other hand, removes parts of the implant material, creating small irregularities and a roughened surface that can encourage rapid osseointegration.9
Though these treatment methods vary, their intended outcome remains the same: To provide a strong biological and mechanical connection to the alveolar bone in a short time period and, ultimately, reduce the likelihood of implant failure.10 Despite the range of implant surfaces being developed over many years, their relative rates of long-term success have only recently been significantly compared.
Study comparing types of dental implant surfaces
A 2018 study, led by Professor Ann Wennerberg, sought to correct this gap in the literature through a systematic review of the long-term clinical outcomes of implant treatment with different surfaces. The study showed that implants with the anodized surface showed the best survival rate (98.5%) with at least 10 years’ follow-up.10
Comparing the performance of implants with anodized, blasted, turned, titanium plasma-sprayed, and sandblasted and acid-etched surfaces, Wennerberg found that there was a mean marginal bone loss for all implant surface types in the study of less than 2 mm, even for implants with older designs and surfaces, and well within what is considered to be an acceptable level.11
Largest ever meta-analysis of a single implant brand
In their 2017 study, Profs. Mattias Karl and Tomas Albrektsson analyzed outcomes from 4,694 clinically evaluated patients treated with 12,803 anodized TiUnite implants reported in 106 studies.12
Their results confirm that implants with the anodized TiUnite surface have a remarkably low early failure rate and very good long-term survival; at implant level, the projected survival rate was over 99% after one year, and 95.1% after 10 years.* As one of the most clinically researched implant surfaces on the market,13 TiUnite has been proven to enhance osseointegration8 and maintain implant stability during the critical healing phase.14 As a result, it is able to play a vital role in assisting clinicians to meet patient demands for shorter time-to-teeth.
Entering the Mucointegration™ era: Developments in anodized surfaces, with Xeal and TiUltra
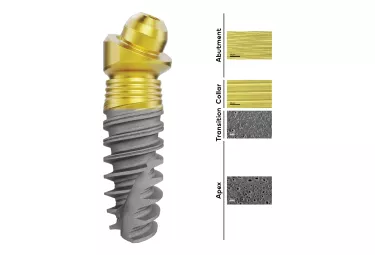
Building on the substantial evidence showing the success of anodized implants, research has explored ways to further utilize this technology. This culminated in the recent introduction of the Xeal and TiUltra surfaces, which apply anodization to the full implant–abutment system.
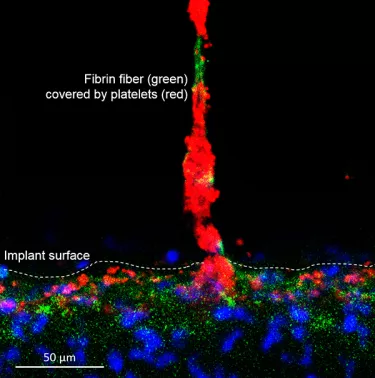
The development of these surfaces looked beyond just microroughness for osseointegration. It investigated ways in which chemistry, nanostructure and porosity/morphology could be tailored to enhance cell attachment, and, to do so at every level – from cortical and cancellous bone osseointegration with the implant, to a soft tissue Mucointegration™ process with the abutment.
Smooth abutment surface for the Mucointegration™ process
Xeal is a smooth, non-porous, nanostructured abutment surface. Dense soft-tissue contact with an abutment can act as a barrier to protect the underlying bone and is the basis for long-term tissue health and stability. With this in mind, Xeal’s surface chemistry and topography were designed to promote soft tissue attachment.15 In clinical and non-clinical studies comparing Xeal-surface abutments with a machined surface, a significantly faster proliferation of human gingival epithelial cells has been observed on the Xeal surface,16 and in a randomized, controlled prospective clinical trial, it demonstrated:
– Significantly higher keratinized mucosa – (2.37mm vs 1.79mm for control (p= 0.024))17
– Significantly lower soft tissue bleeding upon abutment removal (P = 0.006)17
– 4× less bacteria – (primary endpoint, not significant)17
– Stable bone levels17
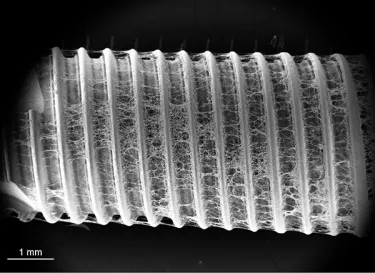
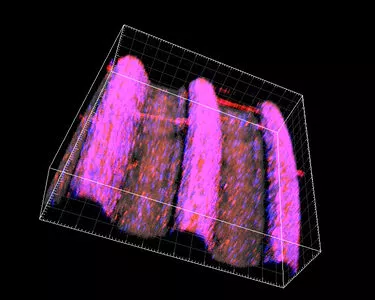
More than roughness – The multi-zone TiUltra™ implant surface
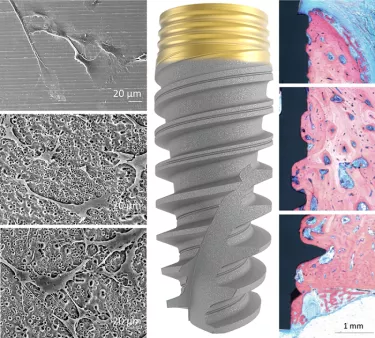
TiUltra is an ultra-hydrophilic, multi-zone surface with a gradual change in topography from collar to apex.15 At collar level it is non-porous, with a nanostructured oxide layer. It then transitions to the apex in terms of roughness, and a low-to-high pore density.15
Studies have shown that human mesenchymal stem cells adhere to and proliferate on each area equally15 (in vitro) and all implant zones osseointegrate with high bone-to-implant contact (in vivo).19
The Xeal abutment surface is available for the On1™ Base and Multi-unit Abutments, and the TiUltra surface on NobelActive®, NobelReplace CC®, and NobelParallel CC™. These surfaces also feature on the new Nobel Biocare N1™ implant system.

Get started with Xeal™ and TiUltra™
Bring Nobel Biocare's most advanced surfaces to your patients. Connect with your local sales representative today.
References
* Results of regression analysis. Details can be found in full publication.
1. Özcan M and Hämmerle C, Titanium as a reconstruction and implant material in dentistry: advantages and pitfalls. Materials. 2012 sep; 5(9): 1528–1545.
Read on PubMed
2. Abraham CM. A brief historical perspective on dental implants, their surface coatings and treatments. Open Dent J 2014;8:50-55.
Read on PubMed
3. Ogle OE. Implant surface material, design, and osseointegration. Dent Clin North Am. 2015;59:505-520.
Read on PubMed
4. Brånemark PI, Zarb GA, Albrektsson T. Tissue-integrated prostheses: Osseointegration in clinical dentistry. Chicago: Quintessence; 1985:201-8.
5. Bagno A, Di Bello C. Surface treatments and roughness properties of Ti-based biomaterials. J Mater Sci Mater Med 2004;15:935–949.
Read on PubMed
6. Sul YT, Johansson CB, Röser K, Albrektsson T. Qualitative and quantitative observations of bone tissue reactions to anodised implants. Biomaterials 2002;23:1809–1817.
Read on PubMed
7. Hall, J. and J. Lausmaa (2000). Properties of a new porous oxide surface on titanium implants. Applied Osseointegration Research 1(1): 5-8.
Read on PubMed
8. Ivanoff, C. J., et al. (2003). Histologic evaluation of bone response to oxidized and turned titanium micro-implants in human jawbone. Int J Oral Maxillofac Implants 18(3): 341-348.
Read on PubMed
9. Barfeie A, Wilson J, Rees J. Implant surface characteristics and their effect on osseointegration. British Dent J 2015;218:1-9.
Read on PubMed
10. Wennerberg A, Albrektsson T, Chrcanovic B. Long-term clinical outcome of implants with different surface modifications. Eur J Oral Implantol 2018;11(supp1): S123–S136.
Read on PubMed
11. Misch CE, et al. Implant success, survival, and failure: the International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent 2008;17(1):5–15.
Read on PubMed
12. Karl M, Albrektsson T. Clinical performance of dental implants with a moderately rough (TiUnite) surface: a meta-analysis of prospective clinical studies. Int J Oral Maxillofac Implants 2017;32(4):717–734.
Read on PubMed
13. TiUnite literature search. Nobel Biocare Services AG. September, 2018.
14. Glauser R, Portmann M, Ruhstaller P, et al. Stability measurements of immediately loaded machined and oxidized implants in the posterior maxilla. A comparative clinical study using resonance frequency analysis. Appl Osseointegration Res 2001;2:27-29.
Access on ResearchGate
15. Milleret V, et al.. Rational design and in vitro characterization of novel dental implant and abutment surfaces for balancing clinical and biological needs. Clin Implant Dent Relat Res. 2019;21 Suppl 1:15-24. doi:10.1111/cid.12736
Read online
16. Nosswitz et al., Evaluation of anodized surfaces designed for improved soft tissue integration. Foundation for Oral Rehabilitation, 2019
Read online
17. Hall J, et al. A randomized, controlled, clinical study on a new titanium oxide abutment surface for improved healing and soft tissue health. Clin Implant Dent Relat Res. 2019;21:e55–e68.
Read online
18. Roffel S, et al. Evaluation of a novel oral mucosa in vitro implantation model for analysis of molecular interactions with dental abutment surfaces, Clin Implant Dent Relat Res 2019;21:e25–e33
Read online
19. Susin C, Finger Stadler A, Musskopf ML, et al. Safety and efficacy of a novel, gradually anodized dental implant surface: A study in Yucatan mini pigs. Clin Implant Dent Relat Res 2019;21(Suppl 1):44-54
Read online