What’s your favorite color? A quick guide to surface anodization
Implant and abutment surface characteristics may be crucial, yet they can be difficult to see with the naked eye. However, the newly developed Xeal and TiUltra surfaces have a distinct appearance. Here’s why.
Surface characteristics are key to the body’s response to the implant and abutment that you place in your patient.1,2 Ultimately, it could decide whether your mission for tissue integration succeeds or fails, both for early healing and long-term stability.3
This is why the surface treatment chosen by your implant and abutment manufacturer is so important: their engineering determines those crucial surface characteristics.
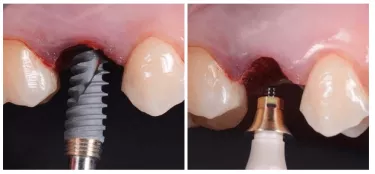
Despite the importance of changes to the surface, to the naked eye, it might be difficult to see such a difference – other than a different shade of gray, depending on the treatment process. However, with the recently developed anodized surfaces, Xeal and TiUltra, you will see a distinctive golden hue.
This coloration has not been created simply for appearance. The gold is a by-product of our advancements in applying this technology, in order to create different characteristics for different tissue integration – from soft tissue, to cortical bone, to cancellous bone. Anodization can take titanium through an entire spectrum of color, depending on the surface characteristics it creates.
What is anodization?
Anodization is an electrochemical process used to engineer a titanium surface.
While titanium provides high-strength and cell adhesion, it’s the oxide layer – instantly created when titanium is exposed to the air – that makes tissue attachment possible.4 While subtractive surface technology (such as sandblasting and/or acid etching) removes material to create the roughness, anodization does the opposite – it increases the thickness of the oxide layer.
And it’s this change of thickness that causes the change of color. The basic process is this: We place the implant in an electrolyte fluid, making it the anode when we apply an electric voltage. As the voltage intensifies, and the length of time increases, the oxide layer expands to a thickness of up to 10,000 nm.5 The oxide’s changing thickness tailors interference of light at the surface, and the thicker it becomes, the more its color moves along the spectrum.
If a critical voltage is reached, sparks appear (spark anodization) and the oxide begins to break down, creating even more roughness with volcano-shaped nodules.6 The color then returns to grey, but with a matt finish.
Why the golden hue?
Our surface treatment of Xeal abutments and TiUltra implant collars has not been tailored simply for appearance. Nonetheless, the color at abutment and implant collar level could potentially bring its own benefits: Studies have shown that improved soft-tissue appearance can be achieved by changing the abutment color from gray to yellow or pink.7-10
But in essence, the golden hue is a consequence of the time and voltage needed to create a surface topography and surface chemistry specifically designed to optimize tissue attachment at a collar and abutment level.
At abutment level, studies have shown that:
– An oxidized, nanostructured surface stimulates more gingival-fibroblast adhesion than machined.11,12
– An oxidized surface enables more epithelial-cell attachment than a machined surface.13,14
– Reduced surface roughness at the abutment can decrease plaque accumulation.15-17
At implant collar level, it is important to minimize marginal bone loss.17 Turned surfaces with just a slight roughness have demonstrated this after over 10 years of function18; and minimal to moderate roughness can reduce marginal bone loss compared to smooth surfaces.19,20
Built on evidence demonstrating the benefits of a smooth, anodized, nanostructured abutment and a minimally rough, anodized, nanostructured implant collar, our applied anodization has been fine-tuned even further. The result? As well as a desired topography and surface chemistry, it’s a surface with a golden hue.
Fine-tuning anodization – it’s more than roughness
Nobel Biocare has two decades’ expertise in applying anodization technology. After the original transition from machined to anodized implants, the impact on early failure rates was truly remarkable; from 11.4% to just 2.1% in the maxilla*.21 When it comes to long-term survival, the anodized surface showed a significantly higher survival rate than surfaces used by other brands for ten years or longer.18 Our further steps forward today go beyond just roughness, but chemistry, ultra-hydrophilicity and protection of the surface too.
References
*Average failure rate of machined implants 1986–2002, compared to anodized TiUnite implants 2003–2011
1. Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10(6):387-416.
Read on PubMed
2. Hall J, Neilands J, Davies JR,Ekestubbe A, Friberg B. A randomized, controlled, clinical study on a new titanium oxide abutment surface for improved healing and soft tissue health. Clin Implant Dent Relat Res.2019;21:55–68.
Read on PubMed
3. Wennerberg A, Albrektsson T. On implant surfaces: a review of current knowledge and opinions. Int J Oral Maxillofac Implants. 2010;25:63-74.
Read on PubMed
4. Abraham CM. A brief historical perspective on dental implants, their surface coatings and treatments. Open Dent J 2014;8:50-55.
Read on PubMed
5. Sul YT, Johansson CB, Petronis S, et al. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: the oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomaterials. 2002;23:491-501.
6. Diamanti MV, Del Curto B, Pedeferri M. Anodic oxidation of titanium: from technical aspects to biomedical applications. J Appl Biomater Biomech. 2011;9:55-69.
7. Wang T, Wang L, Lu Q, Fan Z. Changes in the esthetic, physical, and biological properties of a titanium alloy abutment treated by anodicoxidation. J Prosthet Dent. 2019;121(1):156-165.37.
Read on PubMed
8. Gil MS, Ishikawa-Nagai S, Elani HW, et al. A prospective clinical trial to assess the optical efficacy of pink neck implants and pink abutments on soft tissue esthetics. J Esthet Restor Dent. 2017;29(6):409-415.38.
Read on PubMed
9. Lops D, Stellini E, Sbricoli L, Cea N, Romeo E, Bressan E. Influence of abutment material on peri-implant soft tissues in anterior areas with thin gingival biotype: a multicentric prospective study. Clin Oral Implants Res. 2017;28:1263-1268.
Read on PubMed
10. Guida L, Oliva A, Basile MA, Giordano M, Nastri L, Annunziata M. Human gingival fibroblast functions are stimulated by oxidized nanostructured titanium surfaces. J Dent. 2013;41:900-907.
Read on ResearchGate
11. Wang X, Lu T, Wen J, et al. Selective responses of human gingival fibroblasts and bacteria on carbon fiber reinforced polyetheretherketone with multilevel nanostructured TiO2.Biomaterials. 2016;83:207-218.
Read on PubMed
12. Mussano F, Genova T, Laurenti M, et al. Early response of fibroblasts and epithelial cells to pink-shaded anodized dental implant abutments:an in vitro study.Int J Oral Maxillofac Implants. 2018;33:571-579.
Read on PubMed
13. Nosswitz M , Teale M, Mathes S, Venturato A, Gasser A. Evaluation of anodized surfaces designed for improved soft tissue integration, Foundation for Oral Rehabilitation (FOR) 2019, pp. 1-7.
Read online
14. Elter C, Heuer W, Demling A, et al. Supra- and subgingival biofilm formation on implant abutments with different surface characteristics. IntJ Oral Maxillofac Implants. 2008;23:327-334.
Read on PubMed
15. Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants. 1996;11:169-178.
Read on PubMed
16. Quirynen M, van der Mei HC, Bollen CM, et al. An in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaque. J Dent Res. 1993;72:1304-1309.
Read on PubMed
17. Milleret V, Lienemann PS, Gasser A,Bauer S, Ehrbar M, Wennerberg A. Rational design and in vitrocharacterization of novel dental implant and abutment sur-faces for balancing clinical and biological needs.Clin ImplantDent Relat Res. 2019;21:15–24.
Read online
18. Wennerberg A, Albrektsson T, Chrcanovic B. Long-term clinical outcome of implants with different surface modifications. Eur J Oral Implantol. 2018;11(suppl 1):S123-S136.
Read on PubMed
19. Koodaryan R, Hafezeqoran A. Evaluation of implant collar surfaces for marginal bone loss: a systematic review and meta-analysis. Biomed ResInt. 2016;2016:4987526.
Read on PubMed
20. Mendonca JA, Senna PM, Francischone CE, Francischone Junior CE,de Souza Picorelli Assis NM, Sotto-Maior BS. Retrospective evaluation of the influence of the collar surface topography on peri-implant bone preservation. Int J Oral Maxillofac Implants. 2017;32:858-863
Read on PubMed
21. Jemt T, Olsson M, Franke Stenport V. Incidence of first implant failure: a retro-prospective study of 27 years of implant operations at one specialist clinic. Clin Implant Dent Relat Res 2015;17(Suppl 2):e501-e510.
Read on Pubmed