Ceramic implants come of age
Prof. Holst explores the development of ceramic implants, and how Nobel Biocare now offers a unique solution giving clinicians peace of mind in a niche, yet growing market.

The author serves as Vice President, Global Research, Products and Marketing at Nobel Biocare headquarters in Zurich, Switzerland. Both an experienced clinician and a teacher, he is an adjunct professor at the University of Pennsylvania and an associate professor at the University of Frankfurt.
Here, Professor Holst explores the development of ceramic implants, and how Nobel Biocare now offers a unique solution that can give clinicians peace of mind in this niche, yet growing market.
Substantial growth in our body of knowledge
Zirconia has been used in dental applications for decades – sometimes successfully, sometimes less so. The first zirconia implants were introduced on an experimental basis well before the turn of the century.1 Systems have come and gone. Review the literature or visit a clinic today, and you’ll find that most of the early-phase implants are associated with complications.
We’ve seen a range of problems, including fractures during surgery, fractures after loading, mobility, infection, pain, bone loss, and a lack of osseointegration.2 On the microlevel, inconsistent material quality, less-than-optimal manufacturing processes and surface modifications may bear part of the blame. On the macro level, design flaws have led to unfavorable loading conditions.
At the heart of this apparent conundrum lies a simple fact, well worth remembering: Zirconia is not a metal. Zirconia is a ceramic; and we need to respect the unique properties of this material. Because manufacturing flaws – even minute imperfections – in the production and surface treatment of the zirconia implant may compromise strength, great care must be taken with the materials to ensure clinical predictably and favorable long-term results.3
Consequently, when processing zirconia, it is essential to select the appropriate zirconia powder, the proper powder particles, how to best compact these powders, the ideal postprocessing methods, and so on. Choosing the wrong mix of variables can easily lead to early degradation and failure over time.
Flaws may not manifest themselves immediately, but micro- and macro-flaws with lag times of three to five years have led to the failure of many zirconia implants.
In short, directly applying what we have learned from over 40 years of experience with titanium implants to zirconia does not provide a suitable path forward. In the development of NobelPearl, we have discovered, for instance, that the implant connection design of a zirconia implant cannot be copied one-to-one from existing titanium implants. We’ve also learned a great deal more about the material over recent years.
More than ten years ago, Nobel Biocare sponsored clinical trials of a potential product that was not brought to market. Those trials generated neither the data nor the clinical outcomes we require.4 But over the interceding decade our body of knowledge has grown substantially and researchers with whom we collaborate have now reached the point where we can provide a zirconia implant that is designed to work reliably.
Serious challenges to meeting patient demand
Looking at the zirconia market from a patient perspective, you see increasing demand. Projected growth indicates that although zirconia is very unlikely to replace titanium as the base material for implants anytime soon – if ever – it is already becoming an alternative worthy of attention in certain clinical situations. The intrinsic esthetic attributes of this material are well known. Perhaps less well-known is the fact that this material manifests beneficial soft-tissue-friendly biological properties.5
Zirconia, as an implant material, has provided some serious challenges to innovators over the years. Because it is a brittle material, early attempts to make it stronger involved increasing the volume of the product. As a result, early zirconia implants were predominantly one-piece designs. Unfortunately, such designs have an untoward impact on restorability, placement and intraoral adjustment.
No one wants to face these issues on a day-to-day basis with their patients. Development consequently began on two-piece zirconia implants. They were not easy to handle and attempts at intraoral cementation of the abutment at bone level did not provide the predictable, long-term solution patients deserve.
At least one drawback, however, has been associated with the use of the alternate choice for retention, screws. When patients ask for a metal-free solution, for instance, and you offer them a two-piece zirconia product, can you in good conscience connect the two pieces with a titanium screw? We at Nobel Biocare don’t think so.
Science First
Nobel Biocare has invested in fundamentally understanding the material properties of zirconia and the design limitations these properties impose. In the process, we’ve discovered that there is more to learn from failure than from success. Thus, we studied material science to make the most of the esthetic advantages—while minimizing the mechanical drawbacks—of zirconia.
When you have an implant that’s going into the bone, you want strength, stability, and long-term durability to withstand occlusal forces.
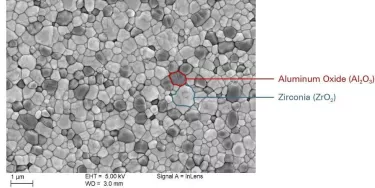
Research has shown us that certain alumina-toughened zirconia (ATZ) compounds, properly prepared, are suitable for this application. In short, ATZ provides the kind of toughness and bending strength we need to facilitate the long-term performance of the implant, especially at the joint interface, where the peak stress forces are concentrated.6
Quality manufacturing
While some manufacturers may be tempted to pursue low-cost injection molding rather than machining – with a relatively high degree of material flaws as a result – NobelPearl instead follows the precise process pathway of cold isostatic pressing, followed by machining, post-compaction and additional grinding.
These manufacturing methodologies have been refined over the past few years in order to ensure the high reliability of the NobelPearl implant.
Altogether, it’s a complicated process, yet one that’s dictated by the properties of the material. To reiterate: For the sake of long-term performance, NobelPearl implants are made out of a fully sintered material via hard machining.
Why do we think that these processes provide significant benefits? To start with, we already know that zirconia osseointegrates properly, just like titanium. 7,8
Thanks to many years of experience with zirconia abutments, it has been possible to develop the right surface roughness and structure for the NobelPearl implant, with the intention of ensuring excellent hard and soft-tissue integration around this new product.9 Also, when it comes to inflammatory responses around these materials, zirconia demonstrates very promising potential.10
The screw joint
The two-piece solution that characterizes NobelPearl, designed with an internal screw-joint connection for restorative flexibility, also eliminates the risks associated with excess cement. NobelPearl consists of high precision manufactured components. In order to ensure that these materials work long-term it might seem natural to choose an existing titanium screw.
However, because titanium is a metal, with very different properties than ceramics, a titanium screw would have an adverse impact on the ceramic interface, which would, in turn, detrimentally affect long-term fatigue strength. This is why this new system includes a carbon-fiber-reinforced polyetheretherketone (PEEK) screw. Carbon-fiber-reinforced polymer is used extensively in the aerospace industry; it’s lightweight, bio-inert, and displays good friction and wear properties.
The contour of a screw made of carbon-fiber-reinforced PEEK adapts to the threads inside the implant, and it does not display the stress concentration typical of metal in contact with zirconia. Simply put, you have more surface contact between PEEK screws and zirconia compared to titanium screws in the same situation. Not only is this to ensure that even with high tensile forces, the abutment will continue to be retrievable, it concomitantly produces a stable concretion joint between the implant and the abutment*.
* May include metal screw or metal insert.
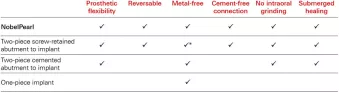
NobelPearl checks all the boxes. What’s more, an internal connection offers restorative flexibility and minimizes risks associate with excess cement.
A complement – not a replacement
NobelPearl has not been designed and developed to replace titanium implants in general, but to provide the clinician with a treatment option in a variety of specific situations. Patients sometimes request alternatives to titanium, for example, for reasons ranging from esthetic considerations to an aversion towards biologically integrated metals. Whatever the reason may be for choosing a zirconia-based solution, NobelPearl provides a response to these concerns in a clinical context.
What more is there to say? As you would expect, long-term clinical studies are in progress. Although we have yet to present five-year data, our experience with zirconia in general, and this design in particular, is so extensive that we are very confident that NobelPearl will prove its long-term reliability for the kinds of cases where a zirconia-based solution is preferred.
This implant system has undergone exactly the same test setup as all our titanium implants (ISO 14801) and has achieved excellent results.* Its internal connection offers a great deal of restorative flexibility and minimizes the risks associated with excess cement that you can face with conventional one-piece zirconia implants.
Over and above that, NobelPearl provides the significant benefits of submerged healing while being reversible and metal-free – and its protocol calls for no intraoral grinding. Last but not least, the two-piece nature of the NobelPearl system means that clinicians can treat patients with a zirconia implant using protocols similar (although not identical) to those with which they are familiar from previous training and practice.
*Data on file GEN 160401
References
1. Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol 2000 2017;73(1):241-58.
2. Osman R, Swain M. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015;8(3):932.
3. Osman R, Swain M. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015;8(3):932.
4. Kohal RJ, Spies BC, Bauer A, Butz F. One-piece zirconia oral implants for single-tooth replacement: Three-year results from a long-term prospective cohort study. J Clin Periodontol 2018;45(1):114-24.
5. Cionca N, Hashim D, Cancela J, Giannopoulou C, Mombelli A. Pro-inflammatory cytokines at zirconia implants and teeth. A cross-sectional assessment. Clin Oral Investig 2016;20(8):2285-91.
6. Spies BC, Sauter C, Wolkewitz M, Kohal RJ. Alumina reinforced zirconia implants: effects of cyclic loading and abutment modification on fracture resistance. Dental Materials 2015;31(3):262-72.
7. Pieralli S, Kohal RJ, Jung RE, Vach K, Spies BC. Clinical Outcomes of Zirconia Dental Implants. J Dent Res 2017;96(1):38-46.
8. Pieralli S, Kohal RJ, Lopez Hernandez E, Doerken S, Spies BC. Osseointegration of zirconia dental implants in animal investigations: A systematic review and meta-analysis. Dental Materials 2018;34(2):171-82.
9. Chappuis V, Cavusoglu Y, Gruber R, et al. Osseointegration of Zirconia in the Presence of Multinucleated Giant Cells. Clin Implant Dent Relat Res 2016;18(4):686-98.
10. Cionca N, Hashim D, Cancela J, Giannopoulou C, Mombelli A. Pro-inflammatory cytokines at zirconia implants and teeth. A cross-sectional assessment. Clin Oral Investig 2016;20(8):2285-91.