The mechanical environment of immediate placement
In this, the second of a three-part series, Dr. Helms discusses our best current understanding of the mechanical context of an immediately loaded implant.
Working with researchers Liao Wang, Xibo Pei, and Yan Wu at the Stanford University Department of Surgery, Professor Jill A. Helms has been studying what happens around immediately loaded implants. In this, the second of a three-part series, Dr. Helms discusses our best current understanding of the mechanical context of an immediately loaded implant.
In the previous issue of Nobel Biocare News we discussed the extraction socket and what happens biologically to the bone tissue there as it heals. In this edition we are going to examine what happens when you place an implant in this setting. In other words, we are going to look closely at the biomechanical interface.
When considering this interface, there are two regions of the extraction socket that are of particular interest.
The first region is where the implant has engaged with the bone. As with traditional placement in an osteotomy, the implant is in direct contact with the bone in this region, and osseointegration tends to follow apace.
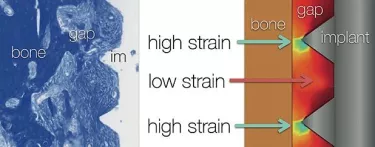
When you put an implant into an extraction socket, however, there is a second region where the implant is not in contact with the bone. Here we see such a gap. (Fig. 1.) Although we call it a “gap,” let me assure you that this choice of word in no way indicates that this space is empty.
The gap we observe between the implant and the extraction wall is filled with tissue. First it fills with blood, then a fibrin clot. The fibrin in turn traps cells, which we model in our laboratory to find out how bone forms in this gap.
My colleague at Stanford, Professor John Brunski, developed a finite element model, which simply and effectively helps us to understand what takes place within this space, filled as it is with blood, fibrin, and other cells.
In order to put Brunski’s finite element model to work in our mouse model, we place an implant in an osteotomy designed to represent the interface we wish to study—the gap region between the bone and the implant. Then we subject the implant to a nominal load, which we do, of course, because we want to study the consequences of immediately loaded implants.
The resulting model is illustrated in Figure 2. We have the implant, the gap filled with the tissue, and the bone, as shown in the left hand panel. Although the gap interface appears uniform, the finite element model shows that there are regions of high strain in this situation. The regions of high strain are around the tips of the implant threads that are near bone.
Please also note the regions of low strain. This turns out to be very, very important. These areas of low strain are, in part, predicated on the design of the implant.
When we look at the regions of high strain, we know that cells cannot differentiate into osteoblasts there if the strain is excessively high (on an order of 30 percent). In regions of low strain, on the other hand, bone formation is actually stimulated.
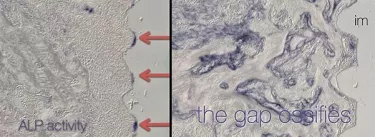
Let’s use a molecular marker to visualize the onset of new bone formation. Using a marker called alkaline phosphatase (ALP), we can clearly observe that it is in the areas of low strain (indicated with the red arrows) where we see the beginnings of new bone formation and mineralization. Gradually, this mineralization spreads and the areas that once represented gaps around the immediate implant ossify (right side of Figure 3).
This work demonstrates what many clinicians have already observed in practice. We can create mechanical environments within the extraction socket where low strain encourages the differentiation of osteoblasts. Of course, encouraging their differentiation—especially in cases where a patient’s own boneforming capacity might be diminished because of age or an underlying health condition—is our ultimate goal.