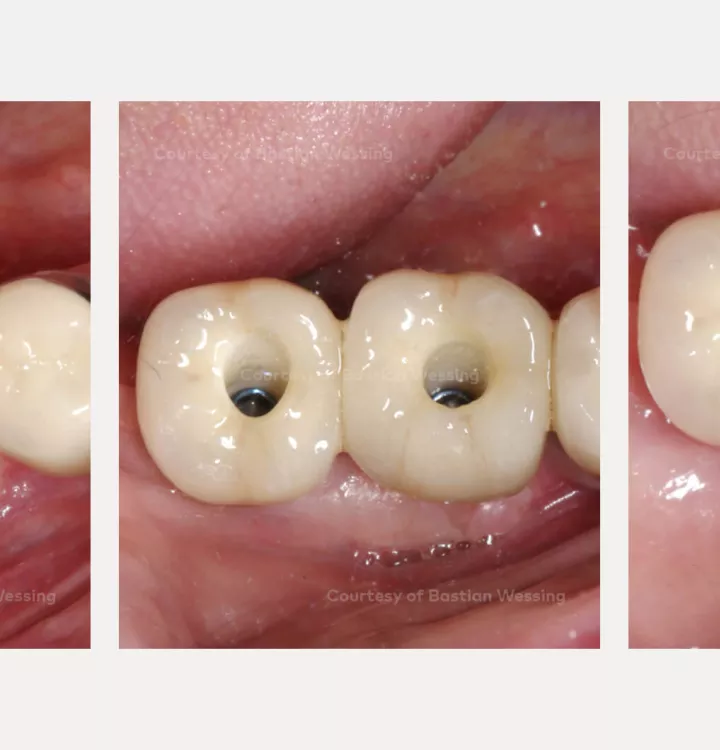
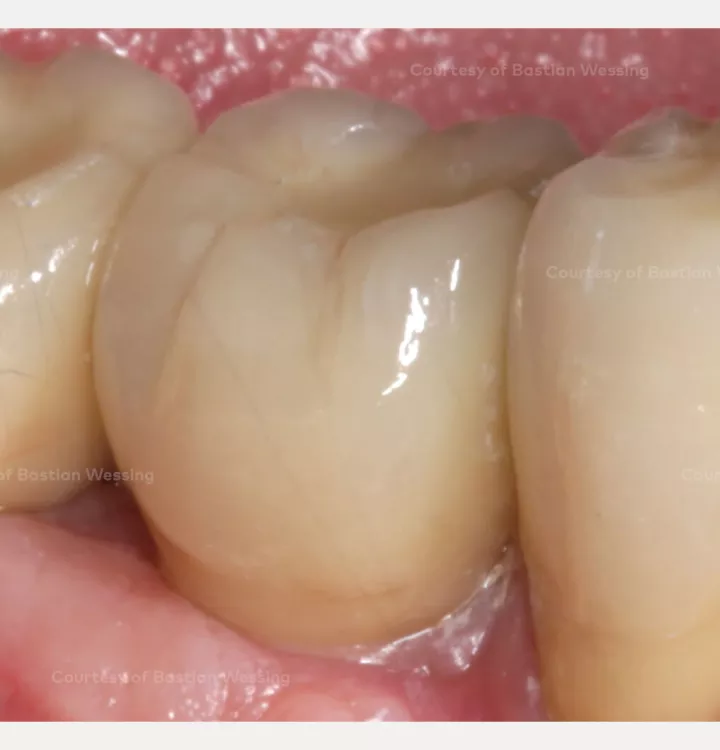
Horizontal and vertical ridge augmentation of a knife-edge ridge
Dr. Bastian Wessing
Germany
“Why do I use the creos™ xenoprotect membrane? Because of the improved mechanical properties…for its effectiveness in many indications.”
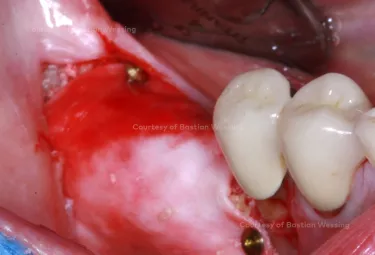
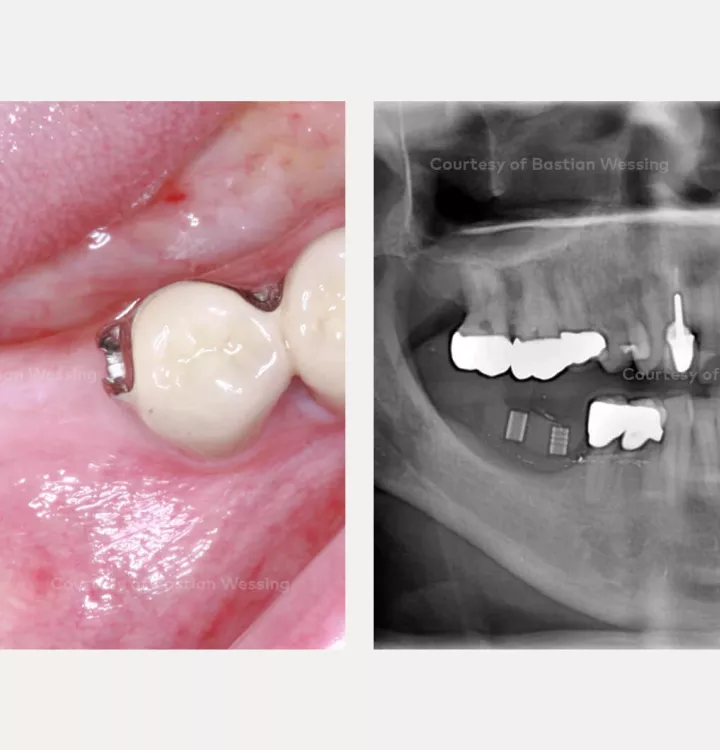
Patient: 54, male
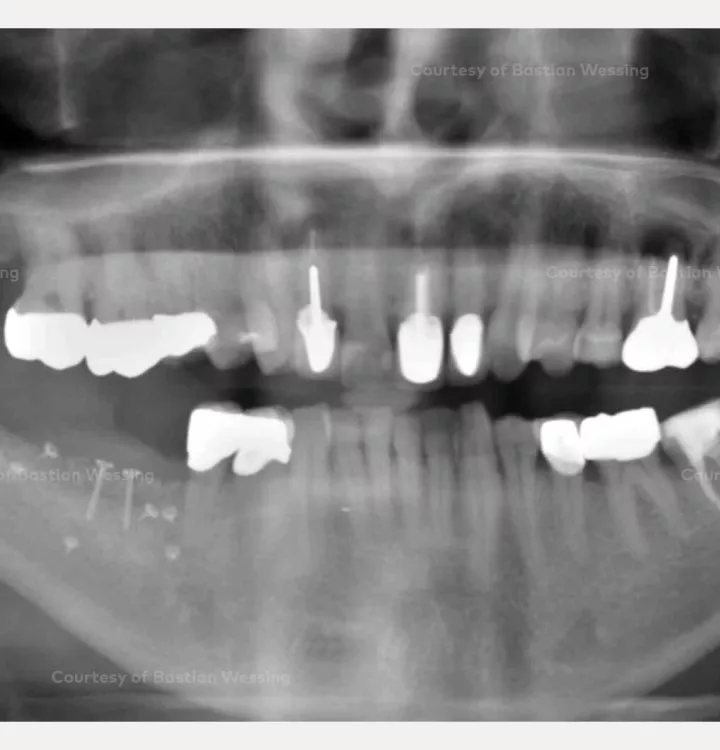
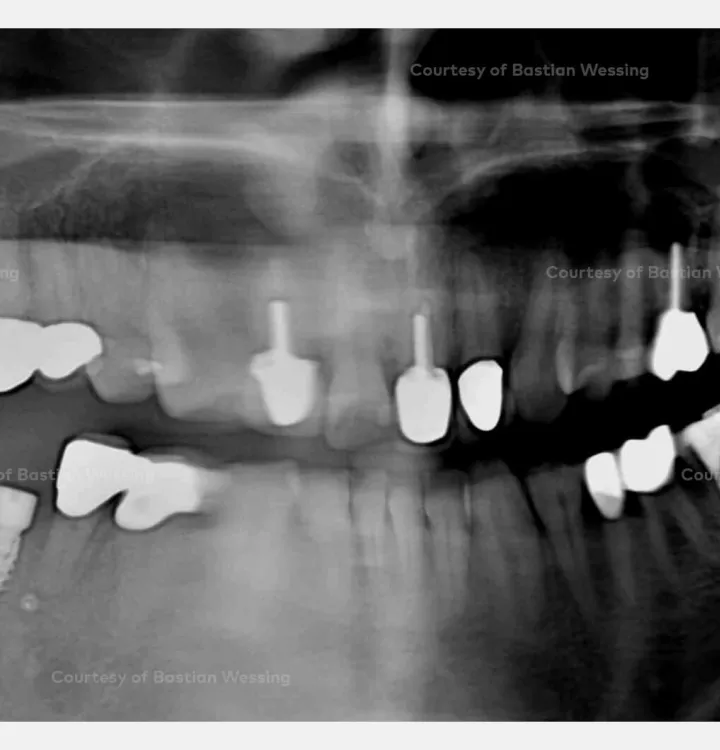
Clinical situation: missing teeth 46, 47. Late implant placement, bone quality D1. Poor bone quantity with 2-3 mm thickness at the crest, 8 mm and 6 mm residual bone on top of the nervus alveolaris inferior in region 46 and 47 respectively.
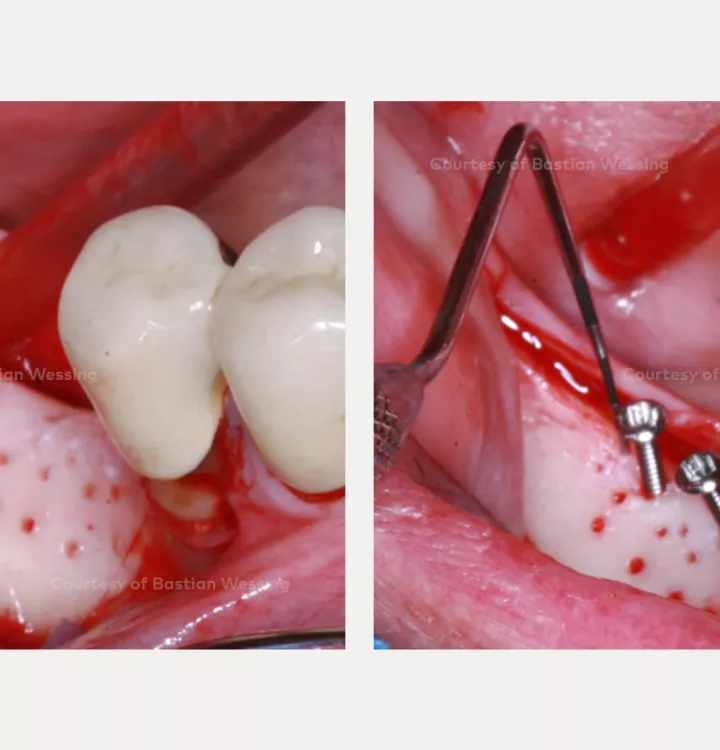
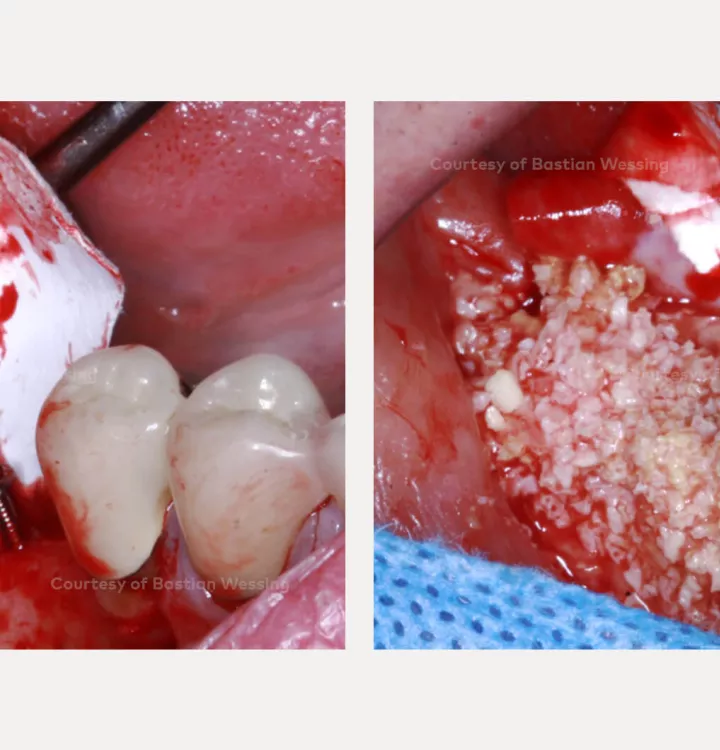
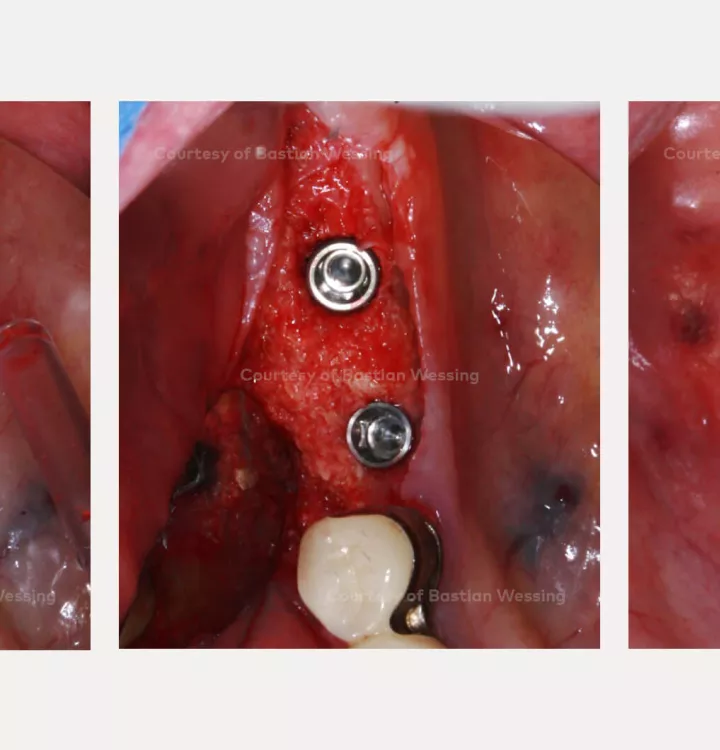
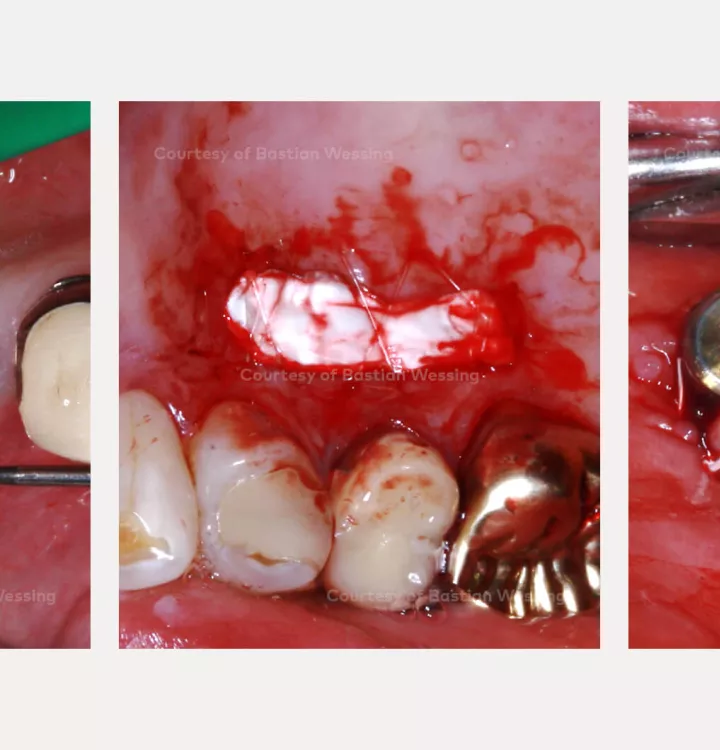
Surgical solution: creos™ xenoprotect membrane. Horizontal and vertical augmentation by GBR using “tenting screw technique”
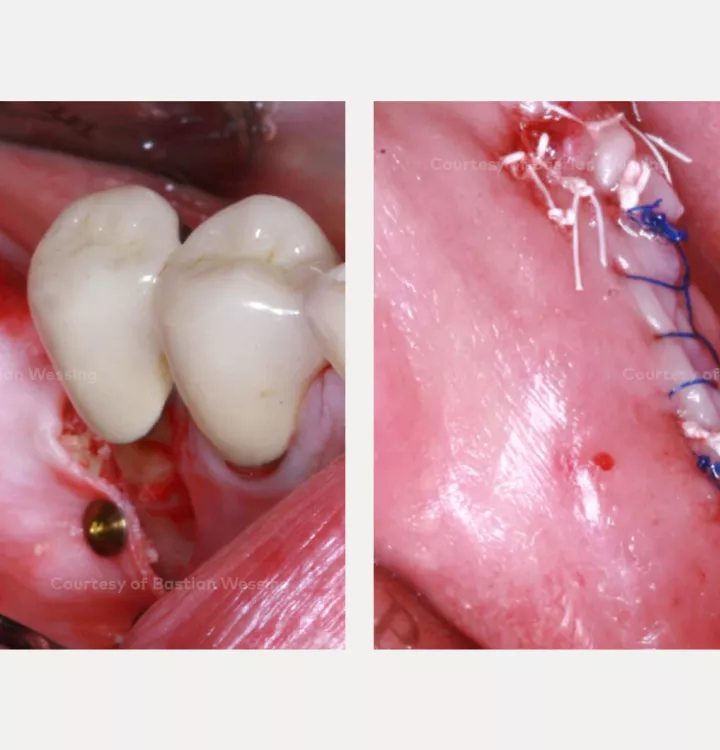
Surgery date: February 25, 2015 (GBR), August 14, 2015 (implant placement), March 18, 2016 (prosthetic restoration)
-
 NobelActive®
NobelActive®An implant like no other.
-
 creos™ xenoprotect
creos™ xenoprotectA membrane with outstanding handling that facilitates bone gain.
Sign up for our blog update
Get the latest clinical cases, industry news, product information and more.
© Nobel Biocare Services AG, 2020. All rights reserved. Nobel Biocare, the Nobel Biocare logotype and all other trademarks are, if nothing else is stated or is evident from the context in a certain case, trademarks of Nobel Biocare. Please refer to nobelbiocare.com/trademarks for more information. Product images are not necessarily to scale. Disclaimer: Some products may not be regulatory cleared/released for sale in all markets. Please contact the local Nobel Biocare sales office for current product assortment and availability. For prescription use only. Caution: Federal (United States) law restricts this device to sale by or on the order of a licensed clinician, medical professional or physician. See Instructions For Use for full prescribing information, including indications, contraindications, warnings and precautions. Nobel Biocare does not take any liability for any injury or damage to any person or property arising from the use of this clinical case. This clinical case is not intended to recommend any measures, techniques, procedures or products, or give advice, and is not a substitute for medical training or your own clinical judgement as a healthcare professional. Viewers should never disregard professional medical advice or delay seeking medical treatment because of something they have seen in this clinical case. Full procedure is not shown. Certain sequences have been cut.