Why abutment surface matters for soft tissue health
As many of us already know, dental implants are designed to facilitate an optimal level of osseointegration. However, there’s another factor that is also of great importance: soft tissue health.
The importance of soft tissue health
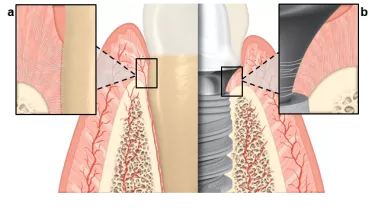
It has been well established that, for healthy teeth, the periodontal soft tissue not only stabilizes teeth, but also creates a barrier between the oral cavity and the body’s inside. The soft tissue’s role is quite similar when it comes to a dental implant: dense soft tissue contact with the abutment surface can act as a barrier that protects and preserves the underlying crestal bone.1,2
However, as Dr. France Lambert pointed out in a previous blog article, the anatomical features of the soft tissue adjacent to a dental implant are different to the soft tissue surrounding natural dentition. Perpendicular collagen fibers known as Sharpey’s fibers connect natural teeth to the cementum, whereas collagen fibers tend to adhere to the abutment surface in a parallel or circumferential manner, which can lead to a weaker connective interface.3
To achieve healthy integration and long-term dental implant success, it is essential to achieve increased mucosal tissue stability surrounding an abutment.4
Two factors that influence soft tissue attachment
It’s fairly common knowledge that the surface of a dental implant can have a significant impact on the implant’s immediate and long-term survival. What has been less frequently interrogated is the role of the abutment surface, and what procedures it should be subject to.
As has been shown in numerous studies, an abutment with a smooth surface not only facilitates mechanical cleaning, but less plaque accumulation has also been observed compared to abutments with a rough surface.4,6-8 However, there are additional elements that need to be considered.
Nanotopography
The nanotopography of a surface has become increasingly important as its role in soft tissue attachment has grown clearer. The surface’s nanostructure is now believed to play a role in promoting cell–implant interactions at a cellular and protein level.9
There are a number of methods for altering the nanotopography of an abutment surface. One such preferred way is anodization, a process which involves submerging the abutment in an electrolytic fluid and applying voltage. This changes the nanotopography that in turn leads to gingival fibroblast adhesion and proliferation, as an important step in soft tissue attachment.10,11
Surface chemistry
This process of anodization also has an effect on the abutment surface chemistry and energy. Research has shown that anodized surfaces have the most hydroxyl groups when compared to sand-blasted and acid-etched surfaces12 and correlates strongly to increased hydrophilicity, or the affinity a surface has for water (or blood).10
It’s also been demonstrated in prior studies that hydrophilic abutment surfaces may assist with adhesion,10-13 supporting gingival soft tissue attachment, which has a functional biological seal for preventing microbial colonization.14
The need for a pristine surface
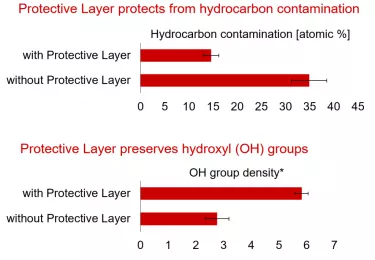
Once any abutment or implant has been manufactured and packaged, atmospheric elements might build up on its surface, even if it is stored in sterile packaging. These deposits tend to have a negative effect on surface energy, which is correlated with hydrophilicity and the abundance of hydroxyl groups.15,16
There is a clear need, then, for the abutment surface to remain in a pristine and unspoiled condition before use, which can be achieved through the use of a protective layer.
Xeal™ – An abutment surface for the Mucointegration™ process4,17,18
Xeal is the latest abutment surface from Nobel Biocare and, together with the TiUltra implant surface, marks the beginning of the Mucointegration™ era. A smooth, non-porous, nanostructured anodized surface, Xeal possesses a surface chemistry and topography that have been designed to achieve soft tissue attachment.19
Though this abutment surface was only introduced to market in 2019, it is already the subject of a clinical study with two years’ follow-up in which it demonstrated a statistically significant increase in keratinized soft tissue height compared to machined abutments.4
In addition to functional benefits, its golden hue (a result of the anodization process) is beneficial in supporting a natural appearance in the transmucosal zone, which may be particularly relevant in cases where thin mucosa or mucosal recession is present.
To ensure that it is in pristine condition, Xeal is also delivered with a Protective Layer that dissolves upon contact with fluid, i.e. blood. This dry packaging technology preserves the abutment surface’s hydrophilicity and surface chemistry and protects it from hydrocarbon contamination.20
Through Xeal and TiUltra, Nobel Biocare’s extensive expertise in anodization technology is applied to the full implant system, from abutment to implant apex.
Conclusion
While the importance of implant surface is common knowledge, abutment surface has undergone far less intense investigation. Dense soft tissue contact with the abutment surface can act as a barrier that protects and preserves the underlying crestal bone needed to achieve healthy integration and long-term dental implant success.4
This has been a driving factor behind the development of the Xeal abutment surface, enhancing the understanding of surface characteristics – especially surface chemistry and nanostructure – to optimize the Mucointegration™ process.
References
1. Al Rezk F, Trimpou G, Lauer HC, Weigl P, Krockow N. Response of soft tissue to different abutment materials with different surface topographies: a review of the literature. Gen Dent 2018 Jan-Feb;66(1):18-25.
2. Gibbs S, Roffel S, Meyer M, Gasser A. Biology of soft tissue repair: gingival epithelium in wound healing and attachment to the tooth and abutment surface. Eur Cell Mater. 2019 Aug 14;38:63-78.
3. Berglundh T, Lindhe J, Ericsson I, Marinello CP, Liljenberg B, Thomsen P. The soft tissue barrier at implants and teeth. Clin Oral Implants 1991;Res 2:81-90.
4. Hall J, Neilands J, Davies JR, et al. A randomized, controlled, clinical study on a new titanium oxide abutment surface for improved healing and soft tissue health. Clin Implant Dent Relat Res 2019;21(Suppl 1):55-68.
5. Nosswitz M, Teale M, Mathes S, Venturato A, Gasser A. Evaluation of anodized surfaces designed for improved soft tissue integration. Foundation for Oral Rehabilitation (FOR) 2019;1-7.
6. Quirynen M, van der Mei HC, Bollen CM, Schotte A, Marechal M, Doornbusch GI, Naert I, Busscher HJ, van Steenberghe D. An in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaque. J Dent Res 1993; 72: 1304-1309.
7. Elter C, Heuer W, Demling A, Hannig M, Heidenblut T, Bach FW, Stiesch-Scholz M. Supra- and subgingival biofilm formation on implant abutments with different surface characteristics. Int J Oral Maxillofac Implants 2008; 23: 327-334.
8. Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants 1996; 11: 169-178.
9. Mendonça G., Mendonça D. B. S., Aragão F. J. L., Cooper L. F. Advancing dental implant surface technology—from micron- to nanotopography. Biomaterials. 2008;29(28):3822–3835.
10. Guida L, Oliva A, Basile MA, Giordano M, Nastri L, Annunziata M. Human gingival fibroblast functions are stimulated by oxidized nanostructured titanium surfaces. J Dent. 2013;41:900-907.
11. Wang X, Lu T, Wen J, et al. Selective responses of human gingival fibroblasts and bacteria on carbon fiber reinforced polyetheretherketone with multilevel nanostructured TiO2. Biomaterials. 2016;83:207-218.
12. Yang Y, Zhou J, Liu X, Zheng M, Yang J, Tan J. Ultraviolet light-treated zirconia with different roughness affects function of human gingival fibroblasts in vitro: The potential surface modification developed from implant to abutment. J Biomed Mater Res Part 8 2015;1038:116-124.
13. Mussano F, Genova T, Laurenti M, Zicola E, Munaron L, Rivolo P, Mandracci P, Carossa S. Early response of fibroblasts and epithelial cells to pink-shaded anodized dental implant abutments: An in vitro study. Int J Oral Maxillofac Implants 2018;33:571-579.
14. Rompen E, Domken O, Degidi M, et al. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: a literature review. Clin Oral Implants Res 2006;17 Suppl 2:55-67.
15. Att W, Hori N, Takeuchi M, Ouyang J, Yang Y, Anpo M, Ogawa T. Time-dependent degradation of titanium osteoconductivity: an implication of biological aging of implant materials. Biomaterials. 2009;30:5352-5363.
16. Hori N, Att W, Ueno T, Sato N, Yamada M, Saruwatari L, Suzuki T, Ogawa T. Age-dependent degradation of the protein adsorption capacity of titanium. J Dent Res. 2009;88:663-667.
17. Susin C, Finger Stadler A, Fiorini T, et al. Safety and efficacy of a novel anodized abutment on soft tissue healing in Yucatan mini-pigs. Clin Implant Dent Relat Res 2019;21(Suppl 1):34-43.
18. Roffel S, Wu G, Nedeljkovic I, et al. Evaluation of a novel oral mucosa in vitro implantation model for analysis of molecular interactions with dental abutment surfaces. Clin Implant Dent Relat Res 2019;21(Suppl 1):25-33.
19. Milleret V, Lienemann PS, Bauer S, Ehrbar M. Quantitative in vitro comparison of the thrombogenicity of commercial dental implants. Clin Implant Dent Relat Res. 2019;21:8–14.
20. Milleret V, Lienemann PS, Gasser A, Bauer S, Ehrbar M, Wennerberg A. Rational design and in vitro characterization of novel dental implant and abutment surfaces for balancing clinical and biological needs. Clin Implant Dent Relat Res 2019;21:e15-e24.