Peri-implantitis and implant surfaces – fact vs. fiction
It can be difficult to distinguish fact from fiction when it comes to implant surfaces and their relationship with peri-implantitis.
Peri-implantitis is a frequent topic of discussion within the dental implant industry and understandably an area of concern for patients. It can be difficult to distinguish fact from fiction when it comes to implant surfaces and their relationship with peri-implantitis. TiUnite is a well-established dental implant surface that has been in use for more than 15 years and has a wealth of evidence behind it. Since the beginning there have been numerous studies to support its performance, and as more studies are published they continue to point to a safe, effective and functional implant surface that offers a number of benefits.
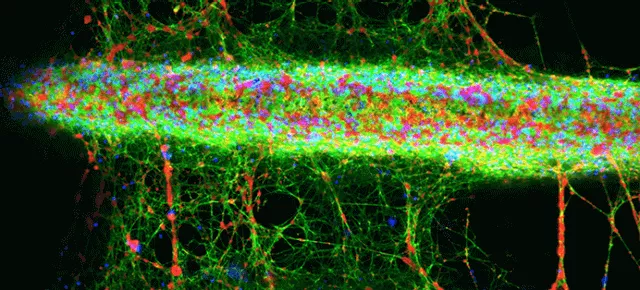
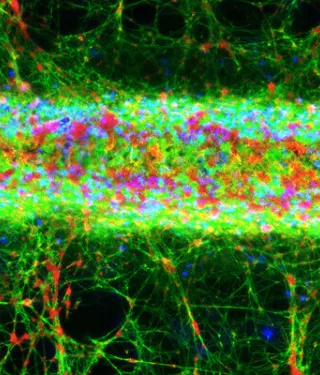
Confocal microscopy z-stack projection shows fluorescent staining of fibrin (green), nuclei of white blood cells (blue) and platelets (red) on a TiUnite surface implant (NobelParallel CC).
What is peri-implantitis?
Peri-implantitis is commonly defined as an inflammatory process affecting the tissues around an osseointegrated implant in function, resulting in the loss of supporting bone.1 It is among the most controversial topics in dentistry, with ongoing debate about whether it can be classified as a disease or should be considered as a complication of placing a foreign body in the oral cavity. Following a recent consensus meeting on peri-implantitis, 17 clinical scientists came to the following conclusions:2
- “Peri-implantitis” is not a clearly defined condition.
- There is no evidence in the literature that a specific peri-implant disease exists as a unique entity with a specific etiology and pathogenesis.
- Progressive marginal bone loss (MBL) after the first year of the implant commonly depends on a complication to treatment that is unrelated to “disease”.
- A one-time radiograph after baseline radiography is insufficient to diagnose “disease”.
- To diagnose peri-implant health, we need a series of radiographs from different times of follow up to decide whether a particular implant has progressive loss of marginal bone or not.
- Periodontal indices such as bleeding on probing and probing depth are not reliable diagnostic tools for identifying “peri-implantitis.”
- There is no clinical evidence published that modern, moderately rough implants will display more “peri-implantitis” than older turned or plasma sprayed implants.
- Peri-implant inflammation is not necessary equal to progressive “disease.”
- Progressive MBL may be caused by numerous factors related to the implant, the clinician, the patient, the treatment protocol, prosthetic overload, quality of care, and maintenance.
- It seems like what we see in cases with truly progressive MBL is related to a complicating factor resulting in an immuno-osteolytic reaction accounting for the ongoing bone resorption
Leading names in the field believe peri-implantitis is often over-reported. Professor Tomas Albrektsson, a pioneer in dental implantology, commented on the need for dental professionals to take a stance against over-reporting of peri-implantitis in the literature:
“I’m increasingly irritated with people calling benign bone loss a disease. Those who are doing so have to read the new research that’s out and realize they are wrong.”
“And the profession must, in a united manner, protest against alarming reports in a much stronger way than we have done to date. But at the same time we must of course continue to take patients very seriously. We cannot ignore bone loss, even if it proves to be benign. We have to be active all the time and work to the best of our knowledge for our patients.”
Importance of the dental implant surface
The mechanical nature of the bone-implant interface is influenced by the nature of the implant surface.3 Different surface characteristics and coatings can greatly affect the longevity and function of a restoration.4
Further to this, implant surface characteristics may have an effect on the development of peri-implant complications, although more clinical studies are needed.5 There are concerns that dissolved titanium could increase the risk of peri-implantitis.6
There are also questions over how well different surfaces can be decontaminated when treating existing peri-implant conditions – for example, chlorhexidine may compromise the biocompatibility of some materials7 and there are some (though again limited) studies to suggest that minimally and moderately rough implant surfaces are associated with less peri-implant bone loss than very rough surfaces.8
The TiUnite dental implant surface was designed to enhance osseointegration, even under the most challenging conditions. It is created by using anodic oxidation to make a thickened layer of moderately rough titanium oxide. In the years since its launch, TiUnite has become one of the most clinically researched implant surfaces ever brought to the market.9 To date, the TiUnite surface has been evaluated in more than 465 publications – comprising over 22,600 patients and 89,500 implants.
4 clinical findings about TiUnite
1. Enhanced osseointegration with fewer failures
Compared with machined surfaces, implants with a TiUnite surface result in stronger and faster osseointegration.10 Following the introduction of the TiUnite surface in 2000, a clear decrease in early implant failures was observed, especially in areas with poor bone density.11 It has been demonstrated that bone grows into the pores of TiUnite, leading to strong interlocking between the surface and the bone.12 This helps to maintain the implant stability achieved at placement throughout the critical healing phase and makes immediate loading possible more frequently, and with superior outcomes.13
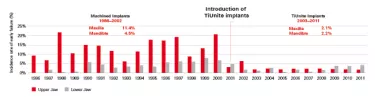
Between 2001 and 2004, the Brånemark clinic in Gothenburg gradually switched from using machined surface implants to those with a TiUnite surface and other moderately rough surfaces. The data tells us that this had a significant impact on early failure rates, which dropped from 11.4% to 2.1% in the maxilla and from 4.5% to 2.2% in the mandible with machined versus TiUnite surface implants, respectively.14
The effect of TiUnite on failure rates is confirmed by Professor Bertil Friberg, who is based at the clinic. He says: “The TiUnite surface has improved our results, especially in grafted bone and in bone of low density. It has, without question, significantly reduced our early failure rate as well.”
2. Stable marginal bone levels
A 10-year study on TiUnite surface implants by Östman et al., reports on 46 partially and fully edentulous patients with 121 TiUnite surface implants.15 While 20% of the implants were immediately loaded, 80% followed a staged protocol.
The research team monitored the patients’ marginal bone levels using intraoral radiographs. They took readings at implant insertion, then after 1 year, 5 years and 10 years of function. Their findings support TiUnite’s performance for offering good clinical outcomes in the long-term.
Östman et al. reports mean marginal bone change was between –0.4 mm at one year, and only –0.3 mm additional bone loss during the subsequent 9 years, and >99% implant survival at 10 years of follow-up.15
3. High survival rates long-term
In addition to the work by Östman et al., mentioned above, further long-term studies have been recorded showing that TiUnite surface implants maintain marginal bone both in partial and full edentulism. This is shown to be the case even following placement in fresh extraction wounds with immediate loading. The cumulative survival rates for these long-term studies are more than 96%.16
4. Low rates of peri-implantitis
In a recent study, researchers found that implants featuring TiUnite provided very good peri-implant soft tissue health, with low rates of peri-implantitis and excellent bone maintenance over 8.5 years.17 The study involved a recall of patients who previously took part in a one-year randomized controlled trial of NobelDirect one-piece implants. The original trial demonstrated a 100% implant survival rate after the first year, and stable marginal bone levels irrespective of whether implants were placed using a flapless or flap protocol.18
At the 8.5-year recall visit, 28 out of 52 patients were available for re-evaluation. Although the NobelDirect implants are no longer commercially available, this data is unique since these one-piece implants feature the TiUnite surface also in the soft tissue tunnel.17
Importantly, the 100% survival rates seen with TiUnite surface implants at 1 year were sustained in patients who were available at this 8.5-year follow-up. Long-term outcomes also remained unaffected by the original placement protocol, with no differences seen between the flapless or flap protocol on any parameter evaluated.18
The low rate of peri-implantitis among patients with TiUnite surface implants was further highlighted in a recent meta-analysis. Researchers evaluated data on 12,803 TiUnite surface implants and 4,694 patients, reporting the rate of peri-implantitis could be as low as 1.36%.19
Conclusions
Overall, implants with the TiUnite surface provide a predictable treatment modality in a variety of indications. They demonstrate excellent peri-implant soft tissue health, bone maintenance and low rates of peri-implantitis. With more than 465 publications – comprising over 22,600 patients and 89,500 implants, it can be stated that implants with the TiUnite surface will provide excellent treatment outcomes for patients.
References
1. Mombelli A. Differential microbial diagnosis and appropriate antimicrobial therapies for peri-implant infections. Association of Dental Implantology 2010. “https://www.adi.org.uk/events/1344/focus_on_periimplantitis/andrea_mombelli” Accessed Oct. 4, 2017.
2. Albrektsson T, Canullo L, Cochran D, et al. Peri-implantitis: a complication of a foreign body or a man-made disease. Facts and fiction. Clini Implant Dent Relat Res 2016;18(4):840-849.
3. Ichim PI, Hu X, Bazen JJ, et al. Design optimization of a radial functionally graded dental implant. J Biomed Mater Res B Appl Biometer 2016;104(1):58-66.
4. Barfeie A, Wilson J, Rees J. Implant surface characteristics and their effect on osseointegration. Br Dent J 2015;218(5):1-9.
5. Renvert S, Polyzois I, Claffey N. How do implant surface characteristics influence peri-implant disease? J Clin Periodontol 2011;38(11):214-222.
6. Safioti LM, Kotsakis GA, Pozhitkov AE,et al.. Increased levels of dissolved titanium are associated with peri-implantitis – A cross-sectional study. J Periodontol 2017;88(5):436-442.
7. Kotsakis GA, Lan C, Barbosa J, et al. Antimicrobial agents used in the treatment of peri-implantitis alter the physicochemistry and cytocompatibility of titanium surfaces. J Periodontol 2016;87(7):809-819.
8. De Bruyn H, Christianes V, Doornewaard R, et al. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontol 2000 2017;73(1):218-227.
9. Karl, M. and Albrektsson, T. Clinical performance of dental implants with a moderately rough (TiUnite) surface: A meta-analysis of prospective clinical studies, Int J Oral Maxillofac Implants 2017;32(4):717-734.
10. Albrektsson T, Johansson C, Lundgren AK, et al. Experimental studies on oxidized implants. A histomorphometrical and biochemical analysis. Applied Osseointegrated Research 2010;1(1):21-24.
11. Balshi SF, Wolfinger GJ, Balshi TJ. Analysis of 164 titanium oxide surface implants in completely edentulous arches for fixed prosthesis anchorage using the pterygomaxillary region. Int J Oral Maxillofac Implants 2005;20:946-952.
Jemt T, Olsson M, Stenport V. Incidence of first implant failure in relation to implant surface: A retroprospective study of 27 years of implant operations at one specialist clinic. Clin Implant Dent Relat Res 2015;17(Suppl 2):e501-e510.
12. Schupbach P, Glauser R, Rocci A, et al. The human bone oxidized titanium implant interface: A light microscopic, scanning electron microscopic, back-scatter scanning electron microscopic, and energy dispersive x-ray study of clinically retrieved dental implants. Clin Implant Dent Relat Res 2005;7(1):36-43.
13. Jungner M, Lundqvist P, Lundgren S. Oxidized titanium implants (Nobel Biocare TiUnite) compared with turned titanium implants (Nobel Biocare Mark III) with respect to implant failure in a group of consecutive patients treated with early functional loading and two-stage protocol. Clin Oral Implants Res 2005;16:308-312.v
14. Jemt T, Olsson M, Stenport FV. Incidence of first implant failure: a retrospective study of 27 years of implant operations at one specialist clinic. Clin Implant Dent Relat Res 2015;17(2):e501-e510.
15. Östman PO, Hellman M, Sennerby L. Ten years later. Results from a prospective single-centre clinical study on 121 oxidized (TiUnite) Brånemark implants in 46 patients. Clin Implant Dent Relat Res 2012;14(6):852-860.
16. Degidi M, Nardi D, Piattelli A. 10-year follow-up of immediately loaded implants with TiUnite Porous Anodized surface. Clin Implant Dent Relat Res 2012;14(6):828-838.
Glauser R. Implants with an oxidized surface placed predominately in soft bone quality and subjected to immediate occlusal loading: results from an 11-year clinical follow-up. Clin Implant Dent Relat Res 2015;18(3):429-438.
17. Froum SJ, Khouly I. Survival rates and bone and soft tissue level changes around one-piece dental implants placed with a flapless or flap protocol: 8.5-year results. Int J Periodontics Restorative Dent 2017;37:326–337.
18. Froum SJ, Cho SC, Elian N, et al. Survival rate of one-piece dental implants placed with a flapless or flap protocol-a randomized, controlled study: 12-month results. Int J Periodontics Restorative Dent 2011;31(6):591-601.
19. Karl, M. and Albrektsson, T. Clinical performance of dental implants with a moderately rough (TiUnite) surface: A meta-analysis of prospective clinical studies, Int J Oral Maxillofac Implants 2017;32(4):717-734.
Share