How do hydrophilic surfaces speed up the healing process?
A considerable number of studies have shown that cells attach better to hydrophilic surfaces than to hydrophobic surfaces.1 But what, exactly, is hydrophilicity? Here, we explain what it is, how it works, and how it can benefit your implant treatment outcomes.
What is hydrophilicity?
Hydrophilicity refers to the affinity that a material has in relation to water. If the contact angle of a water droplet on the surface is less than 90 degrees, that material can be considered hydrophilic.2 Conversely, if the contact angle is more than 90 degrees, it is hydrophobic.
Some surface treatment procedures can make it ‘ultra-hydrophilic’, whereby a drop of water (or blood) spreads and wets the surface when it comes into contact with the surface at close to 0 degrees.3
What causes hydrophilicity?
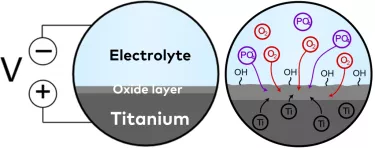
Surface hydrophilicity is determined by surface chemistry, or chemical composition, and procedures exist whereby the chemical composition of a surface can be altered. One example is anodization.
Anodization, briefly put, involves submerging a titanium implant in an electrolytic fluid and applying an electric potential (voltage). This increases the thickness of the titanium oxide layer and alters the topography.4 During anodization, hydroxyl groups, which have been shown to promote bone formation when present on the surface,5-7 are formed in high density. Phosphates, which can increase osteoblastic cell attachment,8 9 can be added to the electrolyte to incorporate them into the oxide layer.
Research has found that, when compared to sand-blasted and acid-etched implants, anodized surfaces have the most hydroxyl groups.10 Furthermore, the surface charge has also been proven to enable higher thrombogenicity (blood clotting) than sand-blasted and acid-etched implant surfaces11; the first of a number of biological processes that enable bone healing.12
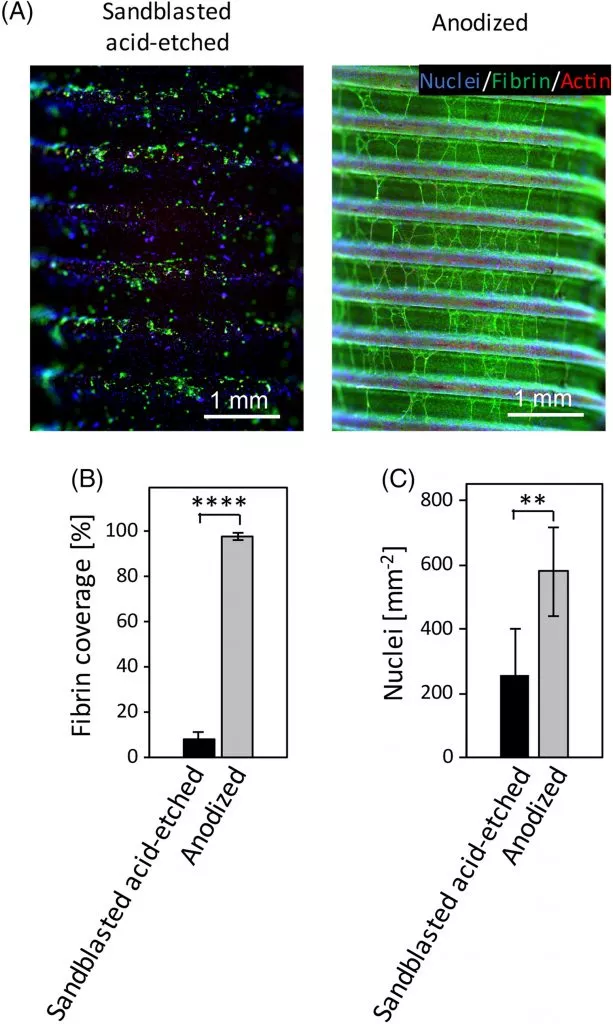
Figure 2: Quantitative in vitro comparison of the thrombogenicity of sandblasted acid-etched and anodized dental implants
Implant surfaces and hydrophilicity
Increasing the hydrophilicity of an implant’s surface can lead to a better interaction between this surface and the surrounding biological environment.13 This increase in activity has a number of benefits for the implant, chief among them being fast osseointegration.14
Hydrophilicity and abutment surfaces
Of course, osseointegration is not the only factor that determines whether a dental implant is successful in the long term.15 Dense soft tissue attachment to the abutment surface is also important in this regard, having been shown to act as a protective seal for the underlying bone against bacterial irritation and inflammation.16-20
A number of studies have demonstrated that hydrophilic abutment surfaces may help to facilitate soft tissue attachment and cell adhesion,21-24 both of which are important factors for long-term implant survival.
Xeal™: A hydrophilic abutment surface for the Mucointegration™ process
Xeal is an anodized abutment surface that, through its surface chemistry and topography properties, has been designed to support soft tissue stability and attachment to the abutment.11 Introduced for the first time in 2019*, this anodized and nanostructured surface is already supported by a clinical study with two-years’ follow-up, the findings of which demonstrate a significant increase in keratinized soft tissue height and better general soft tissue outcomes when compared to a machined surface.25
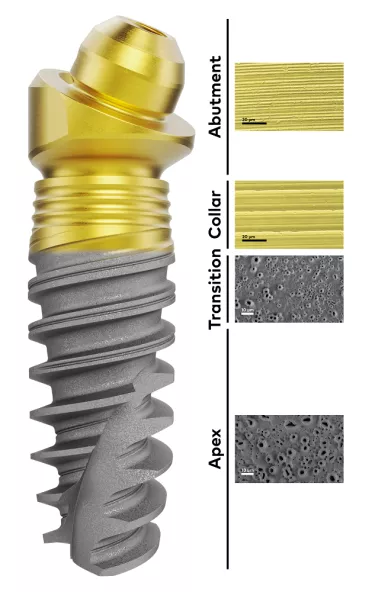
Introduced in parallel with the Xeal abutment surface, TiUltra is an ultra-hydrophilic implant surface, also anodized. The surface chemistry has been designed with the aim of positively influencing its interaction with cells and leading, ultimately, to early osseointegration and long-term bone stability.26 Its multi-zone topography gradually changes from a minimally rough and non-porous collar to a moderately rough and porous implant apex, a transition that respects the natural tooth’s own shift from hard, dense cortical bone to cancellous bone.27
TiUltra™: More than roughness
A preclinical study of TiUltra showed that all areas of the implant surface osseointegrate equally, with high bone-to-implant contact.28
In order to preserve the condition of the surface, Xeal and TiUltra both have a Protective Layer that dissolves upon contact with a fluid, i.e. blood. This layer significantly decreases the amount of carbon build-up on these surfaces, maintaining the surface chemistry allowing for hydrophilicity to be maintained.29
More to explore
– Introducing Xeal and TiUltra: Surfaces cells can’t resist
– What’s your favorite color? A quick guide to surface anodization
– How rough is rough? Why implant surface matters for patients
– 3 things you should know about surface chemistry
References
1. Bauer S, Schmuki A, von der Mark K, Park J. Engineering biocompatible implant surfaces Part I: Materials and surfaces. Progress in Materials Science 2013;58:261–326
2. Albrektsson T, Wennerberg A. On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res 2019;21:4-7
3. Albrektsson T, Wennerberg A. On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res 2019;21:4-7
4. Albrektsson T, Wennerberg A. On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res 2019;21:4-7
5. Fujibayashi S, Neo M, Kim HM, Kokubo T, Nakamura T. Osteoinduction of porous bioactive titanium metal. Biomaterials. 2004;25:443-450.
6. Lai HC, Zhuang LF, Zhang ZY, Wieland M, Liu X. Bone apposition around two different sandblasted, large-grit and acid-etched implant surfaces at sites with coronal circumferential defects: an experimental study in dogs. Clin Oral Implants Res. 2009;20:247-253.
7. Zhao G, Schwartz Z, Wieland M, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74:49-58
8. Park JW, Kim YJ, Jang JH. Enhanced osteoblast response to hydrophilic strontium and/or phosphate ions-incorporated titanium oxide surfaces. Clin Oral Implants Res 2010;21(4):398-408
9. Park JW, Kim YJ, Jang JH, et al. Effects of phosphoric acid treatment of titanium surfaces on surface properties, osteoblast response and removal of torque forces. Acta Biomater 2010;6(4):1661-70.
10. Smeets R, Stadlinger B, Schwarz F, Beck-Broichsitter B, Jung O, Precht C, Kloss F, Gröbe A, Heiland M, Ebker T, Impact of Dental Implant Surface Modifications on Osseointegration, BioMed Research International, vol. 2016, Article ID 6285620, 16 pages, 2016. https://doi.org/10.1155/2016/6285620.
11. Milleret V, Lienemann PS, Bauer S, Ehrbar M. Quantitative in vitro comparison of the thrombogenicity of commercial dental implants. Clin Implant Dent Relat Res. 2019;21:8–14
12. Milleret V, Lienemann PS, Bauer S, Ehrbar M. Quantitative in vitro comparison of the thrombogenicity of commercial dental implants. Clin Implant Dent Relat Res. 2019;21:8–14
13. Sartoretto SC, Alves ATNN, Resende RFB, Calasans-Maia J, Granjeiro JM, Calasans-Maia MD. Early osseointegration driven by the surface chemistry and wettability of dental implants. J Appl Oral Sci 2015 May-June;23(3):279-297.
14. Milleret V, Lienemann PS, Gasser A, Bauer S, Ehrbar M, Wennerberg A. Rational design and in vitro characterization of novel dental implant and abutment surfaces for balancing clinical and biological needs. Clin Implant Dent Relat Res 2019;21:e15-e24.
15. Chrcanovic BR, Albrektsson T, Wennerberg A. Reasons for failures of oral implants. Journal of Oral Rehabilitation 2014;41:443–476.
16. Zheng M, Yang Y, Liu X, Liu M, Zhang X, Wang X, Li H, Tan J. Enhanced biological behavior of in vitro human gingival fibroblasts on cold plasma-treated zirconia. PLoS ONE 2015;10(10):1-17
17. Rompen E, Domken O, Degidi M, et al. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: a literature review. Clin Oral Implants Res 2006;17 Suppl 2:55-67
18. Alva H, Prasad KD, Prasad AD. Bioseal: The physiological and biological barrier for osseointegrated supported prosthesis. J Dent Implant 2013;3:148-52
19. Touati B, Rompen E, Van Dooren E. A new concept for optimizing soft tissue integration. Pract Proced Aesthet Dent. 2005;17(10):711-715
20. Schupbach P, Glauser R. The defense architecture of the human periimplant mucosa: a histological study. J Prosthet Dent. 2007 Jun;97(6 Suppl):S15-25
21. Yang Y, Zhou J, Liu X, Zheng M, Yang J, Tan J. Ultraviolet light-treated zirconia with different roughness affects function of human gingival fibroblasts in vitro: The potential surface modification developed from implant to abutment. J Biomed Mater Res Part 8 2015;1038:116-124
22. Guida L, Oliva A, Basile MA, Giordano M, Nastri L, Annunziata M. Human gingival fibroblast functions are stimulated by oxidized nano-structured titanium surfaces. J Dent 2013;41:900-907
23. Wang X, Lu T, Wen J, Xu L, Zeng D, Wu Q, Cao L, Lin S, Liu X, Jiang X. Selective responses of human gingival fibroblasts and bacteria on carbon fiber reinforced polyether ether ketone with multilevel nanostructured TiO2. Biomaterials 2016;83:207-218
24. Mussano F, Genova T, Laurenti M, Zicola E, Munaron L, Rivolo P, Mandracci P, Carossa S. Early response of fibroblasts and epithelial cells to pink-shaded anodized dental implant abutments: An in vitro study. Int J Oral Maxillofac Implants 2018;33:571-579
25. Hall J, Neilands J, Davies JR, Ekestubbe A, Friberg B. A randomized, controlled, clinical study on a new titanium oxide abutment surface for improved healing and soft tissue health. Clin Implant Dent Relat Res 2019;21:55-68
26. Milleret V, Lienemann PS, Gasser A, Bauer S, Ehrbar M, Wennerberg A. Rational design and in vitro characterization of novel dental implant and abutment surfaces for balancing clinical and biological needs. Clin Implant Dent Relat Res 2019;21:e15-e24
27. Milleret V, Lienemann PS, Gasser A, Bauer S, Ehrbar M, Wennerberg A. Rational design and in vitro characterization of novel dental implant and abutment surfaces for balancing clinical and biological needs. Clin Implant Dent Relat Res 2019;21:e15-e24
28. Susin C, Finger Stadler A, Fiorini T, Musskopf ML, de Sousa Rabelo M, Ramos UD, Fiorini T. Safety and efficacy of a novel, gradually anodized dental implant surface – a study in Yucatan mini pigs. Clin Implant Dent Relat Res. 2019;21:e44–e54.
29. Milleret V, Lienemann PS, Gasser A, Bauer S, Ehrbar M, Wennerberg A. Rational design and in vitro characterization of novel dental implant and abutment surfaces for balancing clinical and biological needs. Clin Implant Dent Relat Res 2019;21:e15-e24