
Preliminary results from a prospective clinical study: Xeal™ and TiUltra™
Preliminary* results from a prospective clinical study into the performance of gradually anodized implant surfaces show evident early success. Outcomes of the study reveal an excellent survival rate, healthy soft tissue response and very high patient satisfaction.
Study
- 61 patients (30 females, 31 males).
- 61 single tooth restorations with NobelActive® TiUltra™ implant and On1™ Base Xeal™
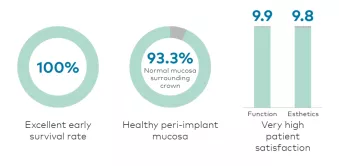
Healthy soft tissue response
- Improved keratinized mucosa status from implant placement to prosthetic delivery.
- Low sulcus bleeding index: 51 sites (85%) showed no bleeding when a periodontal probe was passed along the gingival margin adjacent to the implant.*
- Healthy peri-implant mucosa with 56 sites (93.3%) showing no signs of inflammation surrounding the crown.*
References
*Based on interim analysis by prosthetic delivery, 16.4±7.3 weeks after implant placement.
Ban G and Fabbri G. Clinical study with gradually anodized implants restored with two-piece anodized abutments – preliminary results. Poster presented at: EAO Digital Days 2020, October 5-11.