How rough is rough? Why implant surface matters at every level for patients
It’s the interface between living and inanimate; it’s where biology meets chemistry. Find out why surface matters – and at every level
Implant and abutment surfaces are crucial when it comes to short- and long-term treatment success.
It’s the interface between the living and the inanimate; it’s where the biology meets the chemistry. Ultimately, surface must respect human biology if it’s going to enable soft-tissue attachment to help protect the bone beneath, and to achieve the osseointegration that makes implantology possible.
Why modify the surface?
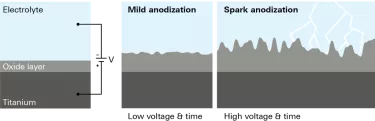
Any implant manufacturer that wants to maximize the likelihood of osseointegration should take the roughness, composition and topography of the surface into account.1
Why are these factors so important? Together, these surface characteristics can contribute to:
– a short healing phase;
– fast osseointegration; and ultimately,
– long-term implant survival.2,3
At Nobel Biocare, our technology of choice is anodization, a process of which we have decades of experience. Using this electrolytic process, we increase the thickness of the titanium oxide layer, change the topography and chemistry of the surface, and ensure the necessary osteoconductivity.
More than roughness
If you ever read about dental implant surface, you will likely see an abundance of the topic of roughness. But important surface characteristics go beyond just roughness, at a macroscopic, microscopic and nanoscopic level.
Macrosurface
Implant thread design is essential for maintaining a high level of primary stability. Studies have shown that an optimal implant thread design should create high stability and greater thread engagement without unnecessary strain.4 For example, NobelActive has a tapered implant body to condense bone gradually, and drilling blades at the apex to allow for a smaller osteotomy.
Microsurface
Key to the success of an implant is to increase its surface area by increasing its porosity.5
This can:
– enhance osteoconductivity;
– lead to fast apposition of newly formed bone; and
– lead to a strong attachment between implant and bone.
The porosity of Nobel Biocare’s anodized, moderately rough surface allows for new bone to form rapidly and provide stronger bone anchorage compared to machined alternatives.6, 7
In fact, a recent systematic review showed that implants with an anodized surface have the best survival rate after 10+ years in function5 compared to other types of surface, such as sand-blasted or machined.
Nanosurface
The nanostructure of a surface (a scale between molecular and microscopic) has become an increasingly important consideration in surface modification. Nanotopography is now thought to influence cell-implant interactions at the cellular and protein level.9

Fig. 2 NobelActive’s expanding tapered implant body condenses bone gradually while the apex with drilling blades enables a smaller osteotomy
Hard- and soft-tissue attachment: Surface matters at every level
Osseointegration may be considered the very foundation of dental implant treatment. However, we should not underestimate the importance of soft-tissue attachment to the abutment.
Why? Soft-tissue attachment creates a barrier for the bone beneath, protecting it from bacteria and the associated tissue inflammation. Furthermore, the smoothness of an abutment surface is expected to allow for control of plaque retention and to facilitate mechanical cleaning.9,10,11
In essence, soft-tissue attachment forms a basis for long-term soft-tissue health and enduring implant stability.
When it comes to hard tissue, we also shouldn’t overlook the fact that the structure and density of the jaw bone aren’t uniform. The bone changes from dense, hard, cortical bone to the spongy, porous and highly vascular interior cancellous bone.
Taking all these tissue levels into account, perhaps surface technology of the future should consider taking a step forward in replicating transitions of tissue with transitions of surface characteristics.


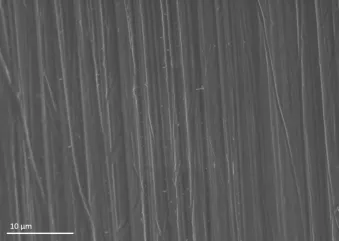
Fig. 4 An abutment surface that is relatively smooth compared to the anodized implant surface. Images © 2018 Nobel Biocare Services AG
What does surface mean for your patients?
Ultimately, there are many benefits for your patients that come from using the right implant and abutment surfaces. A moderately roughened, anodized implant surface, compared to a machined surface, has been shown to offer:
– primary stability needed for immediate loading protocols;5
– promotion of osseointegration; and of
– long-term marginal bone-level preservation12
For the abutment surface, evidence indicates that a smooth abutment surface leads to less accumulation of plaque.10,11,13
Furthermore, the attachment of the soft tissue to the abutment forms a biological barrier, protecting underlying bone for long-term tissue health and stability.
In conclusion, when it comes to implant-system surfaces, the rough and the smooth must work together to achieve successful tissue integration and long-term implant function.
References
1. Koshy E, Philip SR. Dental Implant Surfaces: An Overview. Int J Clin Implant Dent 2015;1(1):14-22.
Read online
2. Maló P, Nobre MDA, Gonçalves Y, Lopes A, Ferro A. Immediate function of anodically oxidized surface implants (TiUnite) for fixed prosthetic rehabilitation: Retrospective study with 10 years of follow-up. Biomed Res Int 2016;1-11.
ResearchGate
3. Sul YT, Johansson CB, Röser K, Albrektsson T. Qualitative and quantitative observations of bone tissue reactions to anodised implants. Biomaterials 2002;23:1809–1817.
Read on PubMed
4. Trisi P, Berardini M, Falco A, Podaliri Vulpiani M. Effect of implant thread geometry on secondary stability, bone density, and bone-to-implant contact: A biomechanical and histological analysis. Implant Dent 2015;24(4):384-391.
Read on PubMed
5. Wennerberg A, Albrektsson T, Chrcanovic B. Long-term clinical outcome of implants with different surface modifications. Eur J Oral Implantol 2018;11(supp1): S123–S136.
Read on PubMed
6. Albrektsson T, Johansson C, Lundgren A-K, et al. Experimental studies on oxidized implants. A histomorphometrical and biomechanical analysis. Appl Osseointegrat Research 2000;1(1):21–24.
7. Omar OM, Lenneras ME, Suska F, et al. The correlation between gene expression of proinflammatory markers and bone formation during osseointegration with titanium implants. Biomaterials 2011;32(2):374–386.
Read on PubMed
8. Mendonça G., Mendonça D. B. S., Aragão F. J. L., Cooper L. F. Advancing dental implant surface technology—from micron- to nanotopography. Biomaterials. 2008;29(28):3822–3835.
Read on PubMed
9. Quirynen M, van der Mei HC, Bollen CM, Schotte A, Marechal M, Doornbusch GI, Naert I, Busscher HJ, van Steenberghe D. An in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaque. J Dent Res 1993; 72: 1304-1309.
Read on PubMed
10. Elter C, Heuer W, Demling A, Hannig M, Heidenblut T, Bach FW, Stiesch-Scholz M. Supra- and subgingival biofilm formation on implant abutments with different surface characteristics. Int J Oral Maxillofac Implants 2008; 23: 327-334.
Read on PubMed
11. Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants 1996; 11: 169-178.
Read on PubMed
12. Karl, M. and Albrektsson, T. Clinical performance of dental implants with a moderately rough (TiUnite) surface: A meta-analysis of prospective clinical studies, Int J Oral Maxillofac Implants. 2017 Jul/Aug;32(4):717-734.
Read on PubMed
13. Bürgers, R, Gerlach, T, Hahnel, S, Schwarz, F, Handel, G, Gosau, M. In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin Oral Implants Res 2010;21:156-164.
Read on PubMed